245 PHOSPHATE ESTER FLAME RETARDANTS
4. CHEMICAL AND PHYSICAL INFORMATION
4.1 CHEMICAL IDENTITY
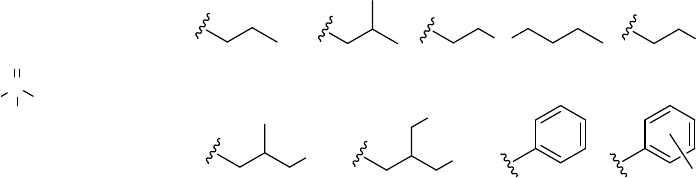
Phosphate esters are considered derivatives of the tri protic acid, phosphoric acid O=P(OH)
3
, with the
general formula of R
x
H
3-x
PO
4
where x=1 for mono, x=2 for di, and x=3 for triesters. Phosphorus has a
high affinity for oxygen due to the difference in electronegativity (1.4), and consequently, the P=O bond
possesses more σ character than π character. Therefore, the P=O bond, which dominates phosphate
chemistry, can be more accurately depicted as a coordinate bond, P→O, or as P
+
–O
-
. These phosphoric
acid esters are often referred to as organophosphates. Trialkyl, triaryl, and trihaloalkyl/aryl, and mixed
phosphate esters possess a central phosphorus atom with an oxidation state of +5 and an approximate
tetrahedral geometry (Fee 2005; Gard 2005).
P
O
R
O
O
R
O
R
R
=
O
C
l
C
l
C
l
C
l
TBP TiBP
TBEP
T
C
EP
T
C
PP TD
C
P TPP
T
C
P
C
H
3
A wide array of substituents can occur as esters of phosphates. In many cases, all of the substituents are
identical, as is the case for this profile; however, variable, mono-, di-, or tri-substituted as well as mixed
substituents are common. The selected compounds, shown above, are trisubstituted, contain identical
substituents, and fall into the following categories: alkyl (TnBP, TiBP), alkyl ether (TBEP), chloroalkyl
(TCEP, TCPP, TDCP), and aryl (TPP, TCP) phosphate esters. Although the majority of selected
compounds are discrete chemicals, commercial formulations of TCPP may contain minor amounts of
structural isomers (NAS 2000). In addition, the commercial mixture of TCP as described here is an
unspecified mixture of isomers, but commercial mixtures are predominantly meta and para isomers with
less than 1% ortho (Winder and Balouet 2002). Table 4-1 lists common synonyms, trade names, and
other pertinent information to identify the selected phosphate esters for this profile.
4.2 PHYSICAL AND CHEMICAL PROPERTIES
Table 4-2 lists important chemical and physical properties of the selected phosphate esters.
246 PHOSPHATE ESTER FLAME RETARDANTS
4. CHEMICAL AND PHYSICAL INFORMATION
Table 4-1. Chemical Identity of Selected Phosphate Ester Flame Retardants
a
Tris(2
-
butoxyethyl)
Characteristic
Tributyl phosphate
Triisobutyl phosphate
phosphate
Synonym(s)
TnBP; butyl phosphate;
TiBP; isobutyl phosphate;
TBEP; tri(2-butoxyethyl)
phosphoric acid tributyl phosphoric acid, phosphate; tributoxyethyl
ester; tri-n-butyl
tris(2-methylpropyl) ester
phosphate;
phosphate; 2-butoxyethanol, phos-
tributoxyphosphine oxide
phate; ethanol, 2-butoxy-,
phosphate (3:1); phos-
phoric acid, tributoxyethyl
ester; tributyl cellosolve
phosphate
Registered trade
Disflamoll TB; Celluphos
No data
Kronitex KP-140; KP 140;
name(s)
4; Phosflex 4
b
; Skydrol
Phosflex T-bep
LD-4
b
Chemical formula
C
12
H
27
O
4
P
C
12
H
27
O
4
P
C
18
H
39
O
7
P
Chemical structure
O
O
P
O
O O
P
O
O
O
O
P
O
O
O
O
O
O
Identification numbers:
CAS registry
RTECS
c
EPA hazardous waste
EPA/OPP pesticide
Code
OHM/TADS
DOT/UN/NA/IMDG
shipping
HSDB
EINECS
NCI
126-73-8
TC7700000
No data
No data
No data
No data
1678
204-800-2
No data
126-71-6
No data
No data
No data
No data
No data
No data
204-798-3
No data
78-51-3
KJ9800000
No data
No data
No data
No data
2564
201-122-9
No data
ula C
18
H
15
O
4
P C
21
H
21
O
4
P C
9
H
18
Cl
3
O
4
P
cture
O
P
O
OO
O
H
3
C
P
OO
O
C
H
3
C
l
O
C
H
3
umbers:
247 PHOSPHATE ESTER FLAME RETARDANTS
4. CHEMICAL AND PHYSICAL INFORMATION
Table 4-1. Chemical Identity of Selected Phosphate Ester Flame Retardants
a
Tri-(2-chloroisopropyl)
Characteristic
Triphenyl phosphate
Tricresyl phosphate
phosphate
Synonym(s)
TPP; phosphoric acid,
TCP; phosphoric acid,
TCPP; tris(1-chloro-
triphenyl ester; tris(methylphenyl) ester; 2-propyl) phosphate;
triphenoxyphosphine
oxide
phosphoric acid, tritolyl
ester; tris(methylphenyl)
tris(2-chloroisopropyl)
phosphate
d
; phosphoric
phosphate
acid, tris(2-chloro-
1-methyl) ether
e
Registered trade
Celluflex TPP; Disflamoll
Kronitex TCP
b
; Phosflex
Hostaflam OP 820;
name(s) TP; Phosflex TPP
179A; Disflamoll TKP;
Lindol; Celluflex 179C
Amgard TMCP; Fyrol
PFC
d
; Antiblaze 80
f
Chemical form
Chemical stru
P
O
O
O
C
l
C
l
Identification n
CAS registry
115-86-6
1330-78-5
13674-84-5
RTECS
c
TC8400000
TD0175000
TC9000000
EPA hazardous waste
No data
No data
No data
EPA/OPP Pesticide
No data
No data
No data
Code
OHM/TADS
No data
No data
No data
DOT/UN/NA/IMDG
IMO 9.0
UN 2574; IMO 6.1
No data
shipping
HSDB
2536
6774
No data
EINECS
204-112-2
215-548-8
237-158-7
NCI
No data
C61041
No data
248 PHOSPHATE ESTER FLAME RETARDANTS
4. CHEMICAL AND PHYSICAL INFORMATION
Table 4-1. Chemical Identity of Selected Phosphate Ester Flame Retardants
a
Tris(2-chloroethyl)
Characteristic
Tris(1,3-dichloro-2-propyl) phosphate
phosphate
Synonym(s)
TDCP; tris(1,3-dichloroisopropyl) phosphate;
TCEP; trichlorethyl
tris(1-chloromethyl-2-chloroethyl)phosphate; phosphate; phosphoric
2-propanol, 1,3-dichloro-, phosphate (3:1)
acid; tris(2-chloroethyl)-
ester; tri(2-chloroethyl)
phosphate; ethanol,
2-chloro-, phosphate
(3:1); tris(2-chloroethyl)
orthophosphate
Registered trade
Fyrol FR-2; Antiblaze 195
f
Antiblaze 100; Celluflex
name(s)
CEF; Disflamoll TCA;
Fyrol CEF; Niax 3CF,
Tolgard TCEP; Genomoll
P; Hostaflam UP810;
Levagard EP
Chemical formula
C
9
H
15
Cl
6
O
4
P
C
6
H
12
Cl
3
O
4
P
Chemical structure
O
C
l
C
l
O
P
O
O
O
C
l
C
l
P
O
O
O
C
l
C
l
C
l
C
l
C
l
Identification numbers:
CAS registry
13674-87-8
115-96-8
RTECS
c
No data
KK2450000
EPA hazardous waste
No data
No data
EPA/OPP Pesticide
No data
No data
Code
OHM/TADS
No data
No data
DOT/UN/NA/IMDG
No data
UN: 3082
g
shipping
HSDB
4364
2577
EINECS
237-159-2
204-118-5
NCI
No data
C60128
a
All information obtained from HSDB 2009, 2011 and ChemIDplus 2009, 2011, except where noted.
b
IPCS 1990,1991a, 2000b.
c
RTECS 2009.
d
Ashford 1994.
e
Lewis 2000.
f
Weil 2001.
g
NIOSH 2007.
CAS = Chemical Abstracts Service; DOT/UN/NA/IMDG = Department of Transportation/United Nations/North
America/Intergovernmental Maritime Dangerous Goods Code; EINECS = European Inventory of Existing Chemical
Substances; EPA = Environmental Protection Agency; HSDB = Hazardous Substances Data Bank; NCI = National
Cancer Institute; NIOSH = National Institute for Occupational Safety and Health; OHM/TADS = Oil and Hazardous
Materials/Technical Assistance Data System; RTECS = Registry of Toxic Effects of Chemical Substances

249 PHOSPHATE ESTER FLAME RETARDANTS
4. CHEMICAL AND PHYSICAL INFORMATION
Table 4-2. Physical and Chemical Properties of Selected Phosphate Ester Flame
Retardants
a
Property
Tributyl phosphate
(TnBP)
Triisobutyl phosphate
(TiBP)
Tris(2-butoxyethyl)
phosphate (TBEP)
Molecular weight
266.31
266.31
c
398.48
Physical
description
Colorless to pale-yellow
liquid
Clear, colorless, low
viscosity liquid
c
Slightly yellow, oily liquid
Melting point
Boiling point
Density
-80 °C
289 °C; decomposes
b
0.9727 g/cm
3
at 25 °C
No data
264 °C
d
0.9681 g/cm
3
at 20 °C
d
-70 °C
215–228 °C at 4 mm Hg
1.020 g/cm
3
at 20 °C
Odor
Odorless
Specific odor
c
Sweetish, butyl-like
Solubility:
Water
0.28 g/L at 25 °C
Very soluble in water
c
;
0.05% in water
c
and 6.3%
water in TnBP
c
1.1 g/L at 25 °C
Organic
solvent(s)
Soluble in diethyl ether,
benzene, carbon disulfide;
miscible with ethanol
Very soluble in benzene,
ether, and ethanol
d
Soluble in most organic
liquids; soluble in mineral
oil; insoluble or limited
solubility in glycerol, glycols,
certain amines
Other
Miscible with most solvents
No data
No data
and diluents
Log K
ow
Vapor pressure
Autoignition
temperature
4.00
1.13x10
-3
mm Hg at 25 °C
>482 °C
b
3.60 (estimated)
e
0.0128 mm Hg at 25 °C
(estimated)
e
No data
3.75
0.03 mm Hg at 150 °C
No data
Flashpoint
146 °C
175 °C (Cleveland)
c
223 °C
Flammability
limits in air
Combustible
No data
Combustible
Conversion
factors
1 ppm=10.89 mg/m
3b
No data
No data
Explosive limits
No data
No data
No data

250 PHOSPHATE ESTER FLAME RETARDANTS
4. CHEMICAL AND PHYSICAL INFORMATION
Table 4-2. Physical and Chemical Properties of Selected Phosphate Ester Flame
Retardants
a
Triphenyl
Tricresyl phosphate
Tri-(2-chloroisopropyl)
Property
phosphate (TPP)
(TCP)
phosphate (TCPP)
Molecular weight
326.28
368.36
327.57
Physical
Description
Colorless, crystalline
powder; white
Colorless liquid
f
; Oily flame
resistant liquid
g
Colorless liquid
f
platelets, crystals
from absolute alcohol-
ligroin, prisms from
alcohol, needles from
ether-ligroin
Melting point
49–50 °C
-33 °C
h
-40 °C
Boiling point
245 °C at 11 mm Hg
265 °C at 10 mm Hg
g
>270 °C; gradually decomposes
when heated over 200°C
f
Density
1.2055 g/cm
3
at 50 °C
1.162 g/cm
3
at 25 °C
1.29 g/cm
3
at 25 °C
f
Odor
Slightly aromatic odor
resembling phenol
Odorless; very slightly
aromatic
h
Mild odor
f
Solubility:
Water
0.0019 g/L at 25 °C
0.00036 g/L at 25 °C
1.2 g/L at 25 °C
Organic
Very soluble in carbon
Miscible with all the common
Soluble in most organic solvents;
solvents
tetrachloride; soluble
solvents and thinners insoluble in water
f
in alcohol, benzene,
ether, chloroform and
acetone; insoluble in
petroleum
Other
Soluble in most
Miscible with vegetable oil;
No data
lacquers, solvents Miscible with lindseed oil,
thinners, and oils
china wood oil, castor oil
g
Log K
ow
4.59
5.11
2.59
Vapor pressure
6.28x10
-6
mm Hg at
6.00x10
-7
mm Hg at 25 °C
2.02x10
-5
mm Hg at 25 °C
25 °C
(extrapolated)
i
Autoignition
No data
No data
No data
temperature
Flashpoint
220 °C
257 °C
h
No data
Flammability
Noncombustible
No data
No data
limits in air
Conversion
1 ppm=13.32 mg/m
3
No data
No data
factors
Explosive limits
No data
No data
No data

251 PHOSPHATE ESTER FLAME RETARDANTS
4. CHEMICAL AND PHYSICAL INFORMATION
Table 4-2. Physical and Chemical Properties of Selected Phosphate Ester Flame
Retardants
a
Tris(2-chloroethyl) phosphate
Property
Tris(1,3-dichloro-2-propyl) phosphate (TDCP)
(TCEP)
Molecular weight
430.88
285.50
Physical
Viscous, clear liquid
Clear, transparent, Low viscosity
Description
liquid
Melting point
27 °C
-55 °C
Boiling point
236–237 °C at 5 mm Hg
330 °C at 1 atm
Density
1.48 g/cm
3
at 25 °C
1.425 g/cm
3
at 20 °C
Odor
Mild odor
Slight odor
Solubility:
Water
7 mg/L at 24 °C
7.0 g/L (temperature not
specified)
Organic
Soluble in most organic solvents
Soluble in most organic solvents;
solvents
soluble in carbon tetrachloride,
alcohols, esters, ketones, and
aromatic hydrocarbons; very
slightly soluble in aliphatic
hydrocarbons; insoluble in
benzene
Other
No data
No data
Log K
ow
3.65
1.44
Vapor pressure
5.2 x10
-2
mm Hg at 25 °C (estimated)
e
6.125x10
-2
mm Hg at 25 °C
Autoignition
No data
1,115 °C
temperature
Flashpoint
252 °C
216 °C
Flammability
No data
Combustible
limits in air
Conversion
No data
1 ppm=11.65 mg/m
3
factors
Explosive limits
No data
No data
a
All information obtained from HSDB 2009, 2011 and ChemIDplus 2009, 2011, except where noted.
b
NIOSH 2005a.
c
LANXESS 2005.
d
Lide 2008.
e
EPA 2009h.
f
Ashford 1994.
g
O’Neil et al. 2006.
h
IPCS 1990.
i
Boethling and Cooper 1985.
