Phosphoric Acid
Handling/Processing
___________________________________
August 4, 2021 Technical Evaluation Report Page 1 of 18
Compiled by Savan Group for the USDA National Organic Program
1
Identification of Petitioned Substance 2
Chemical Names: 3
Phosphoric acid 4
Orthophosphoric acid 5
Polyphosphoric acids 6
Metaphosphoric acid 7
8
Other Name: 9
Phosphoric (V) acid 10
Pyrophosphoric acid 11
Tripolyphosphoric acid 12
Triphosphoric acid 13
14
Trade Names: 15
Phosphoric acid solution 16
CAS Numbers:
Phosphoric acid/orthophosphoric acid: 7664-38-2
Pyrophosphoric acid: 2466-09-3
Triphosphoric acid: 10380-08-2
Metaphosphoric acid: 37267-86-0
Polyphosphoric acid: 8017-16-1
Other Codes:
EC No. (orthophosphoric acid): 231-011-00-6
EC No. (pyrophosphoric acid): 219-574-0
EC No. (triphosphoric acid): 233-840-3
EC No. (metaphosphoric acid): 253-433-4
EC No. (polyphosphoric acid): 232-417-0
17
Summary of Petitioned Use 18
19
In 2019, Kemin Food Technologies petitioned the United States Department of Agriculture (USDA) 20
National Organic Program (NOP) to amend the existing annotation of phosphoric acid on the National List 21
to include use as a synthetic substance for organic processing and handling (USDA 2019, USDA 2020a, 22
USDA 2020b). This new petition requests the expansion of the use of phosphoric acid “as an acidifier to 23
adjust pH of an extraction solvent to extract antioxidants or other target molecules from lamiaceae plants, 24
provided the amount of acid used shall not exceed the minimum needed to lower pH to 2.5” (USDA 25
2020b). In response to the petition by Kemin Food Technologies, the NOSB Materials Subcommittee has 26
requested a technical report focused on the use of phosphoric acid for pH adjustment in the extraction of 27
target compounds from aquatic plants for organic processing and handling. 28
29
In 2002, Aquatic Seaplants Limited petitioned the USDA NOP to expand the approved use of phosphoric 30
acid within the National List to include production of organic aquatic plant extracts (USDA 2002). A 31
technical report on phosphoric acid for organic processing was submitted in 2003 (USDA 2003). In 2004 the 32
NOP contacted the petitioner and stated that phosphoric acid did not need to be petitioned for use in plant 33
extraction “because its use as a pH adjuster in aquatic plant extracts is currently not prohibited through the 34
inclusion of “aquatic plant extracts” in section 205.601(j)(1) of the National Organic Standards” (NOP 2013). 35
In 2013 the NOP sent a memorandum to the National Organic Standards Board (NOSB) requesting a 36
review on the use of phosphoric acid in plant extracts to ensure that this use is consistent with the context 37
to the National List (NOP 2013). 38
39
Characterization of Petitioned Substance 40
41
Composition of the Substance: 42
Orthophosphoric acid is the most common phosphoric acid used in plant extraction applications and is also 43
generically referred to as phosphoric acid (Silberberg 2003, USDA 2003, Shriver and Atkins 2008, Timberlake 44
2016). Due to the predominance of orthophosphoric acid among the many forms of phosphoric acid, the term 45
phosphoric acid will be used to describe orthophosphoric acid throughout the remainder of this report, unless 46
otherwise stated. 47
48
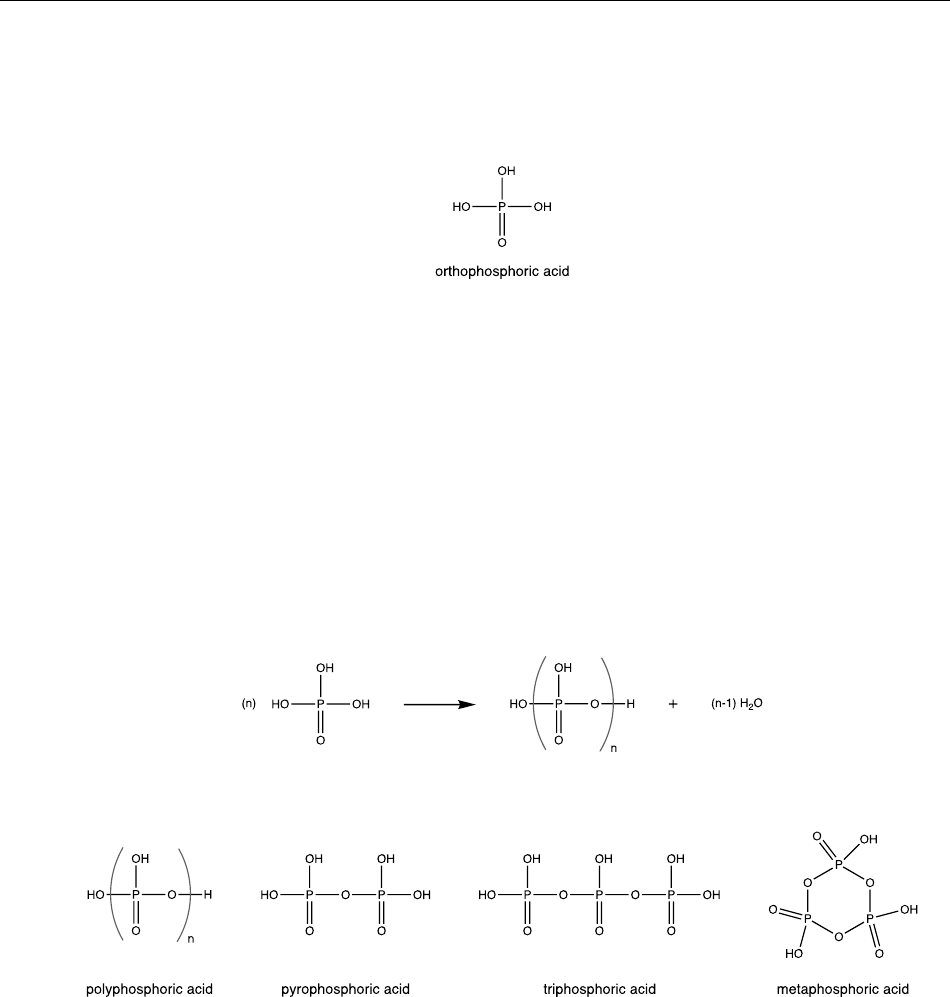
Technical Evaluation Report Phosphoric acid Handling/Processing
August 4, 2021 Page 2 of 18
Phosphoric acid describes several different substances, all of which feature a tetrahedral phosphorous (V) atom 49
surrounded by oxygen atoms (Shriver and Atkins 2008, Gilmour 2019). Phosphoric acid and phosphate 50
compounds are often characterized based on their P
2
O
5
content, which is based on the empirical formula of 51
molecular phosphorus (V) oxide (P
4
O
10
) (Shriver and Atkins 2008, Gilmour 2019). Phosphoric acid may take the 52
form of a single phosphorous atom as orthophosphoric acid (H
3
PO
4
), as shown in Figure 1. 53
54
55
56
Figure 1 57
58
Phosphoric acid molecules polymerize via dehydration to form a variety of polymeric phosphoric acids, known 59
generally as polyphosphoric acids, as shown below in Equation 1 (Shriver and Atkins 2008, Gilmour 2019). 60
Polyphosphoric acids are linked together through phosphoester linkages (P – O – P bond arrangements), as 61
shown below in Figure 2 (Silberberg 2003, Shriver and Atkins 2008, Timberlake 2016, Gilmour 2019). 62
Pyrophosphoric acid (H
4
P
2
O
7
), formed through the polymerization of two phosphoric acid monomers, and 63
triphosphoric acid (H
5
P
3
O
10
), formed through the polymerization of three phosphoric acid monomers, are 64
common polyphosphoric acids and are shown below in Figure 2. In addition to linear polymers, phosphoric acid 65
monomers may combine to form cyclic structures (Gilmour 2019). Metaphosphoric acid is the tri-cyclic form of 66
phosphoric acid (H
3
P
3
O
9
, although it is often listed by its empirical formula, H
3
PO
4
), shown below in Figure 2, 67
however, the term metaphosphoric acid has also been applied generally to describe cyclic phosphoric acids 68
(Shriver and Atkins 2008, Gilmour 2019, SA 2020a). 69
70
71
72
Equation 1 73
74
75
76
Figure 2 77
78
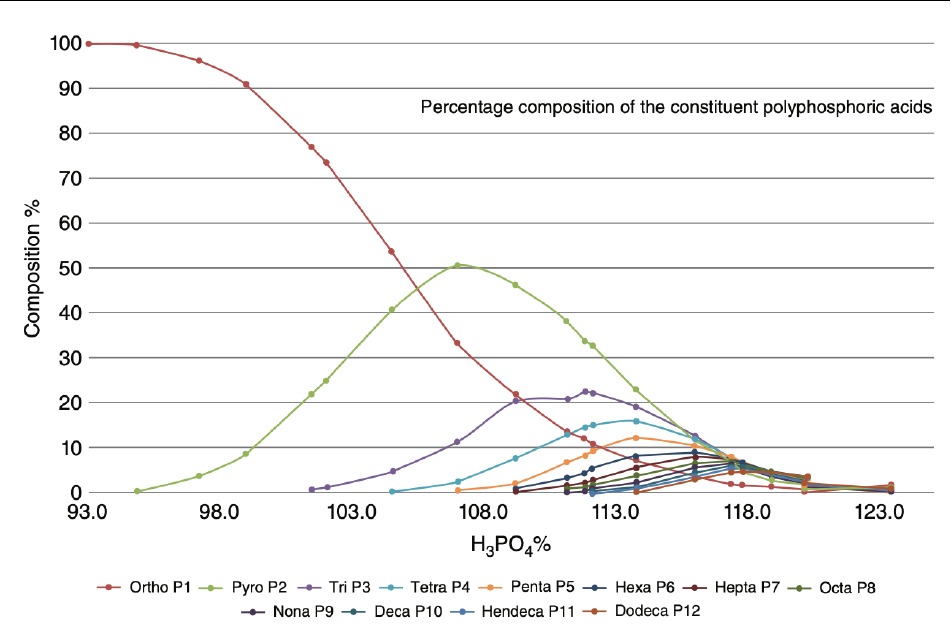
The composition and form of phosphoric acid is dependent on its concentration in solution, as described in the 79
plot below in Figure 3 (Gilmour 2019). At relatively low concentrations of H
3
PO
4
(~94%, [68% P
2
O
5
]) 80
orthophosphoric acid is the predominant form. However, as the concentration of H
3
PO
4
increases, 81
polymerization to polyphosphoric acids becomes more prevalent, and the condensed forms of phosphoric acid 82
become the majority of the species in solution (Gilmour 2019). The P
2
O
5
content of phosphoric acid dictates its 83
physical properties, including appearance, viscosity, and boiling point. Phosphoric acid exists as an oily 84
substance with P
2
O
5
concentration between 72 and 82%. It becomes more viscous with P
2
O
5
composition from 82 85
to 90%. It solidifies when P
2
O
5
composition exceeds 90% (Gilmour 2019). 86
87
Technical Evaluation Report Phosphoric acid Handling/Processing
August 4, 2021 Page 3 of 18
88
89
Figure 3 90
91
All phosphoric acids are weak inorganic acids that are polyprotic (capable of producing multiple acidic units 92
[H
+
]) (Silberberg 2003, Shriver and Atkins 2008, Timberlake 2016, Kalka 2021). The classification of phosphoric 93
acid as a weak acid is based on its incomplete ionization in water, however, concentrated phosphoric acid is a 94
highly acidic and corrosive substance (Silberberg 2003, Shriver and Atkins 2008). Pure phosphoric acid is a solid, 95
although most phosphoric acid exists as an aqueous solution (PC 983, PC 1004, PC 1023, PC 3084658, Gilmour 96
2019, SA 2020a, SA 2020b, SA 2020c, SA 2021). The acidic nature of phosphoric acid results in its reaction with 97
water in aqueous solutions to produce phosphate ions (see Equations 2-4) (Silberberg 2003, Shriver and Atkins 98
2008, Timberlake 2016, Kalka 2021). The multiple equilibria for these acid-base reactions are illustrated with the 99
three ionization reactions possible for phosphoric acid, along with their equilibrium constants (K
a
) and their 100
relative strengths (pKa), shown below in Equations 2 – 4 (Silberberg 2003, Shriver and Atkins 2008, Timberlake 101
2016, Kalka 2021). As with all polyprotic acids, the initial dissociation is the most favorable, with subsequent 102
compounds being less acidic (i.e., acid strength H
3
PO
4
> H
2
PO
4
-
> HPO
4
2-
), as shown with the decreasing 103
equilibrium constants and increasing pKa values in Equations 2 – 4 (Silberberg 2003, Shriver and Atkins 2008, 104
Timberlake 2016, Kalka 2021). 105
106
H
3
PO
4
(aq)
+ H
2
O
(l)
⇌ H
2
PO
4
-
(aq)
+ H
3
O
+
(aq)
K
a1
= 7.2 × 10
-3
(pKa = 2.15) 107
108
Equation 2 109
110
H
2
PO
4
-
(aq)
+ H
2
O
(l)
⇌ HPO
4
2-
(aq)
+ H
3
O
+
(aq)
K
a2
= 6.3 × 10
-8
(pKa = 7.12) 111
112
Equation 3 113
114
HPO
4
2-
(aq)
+ H
2
O
(l)
⇌ PO
4
3-
(aq)
+ H
3
O
+
(aq)
K
a3
= 4.2 × 10
-13
(pKa = 12.35) 115
116
Equation 4 117
118
Technical Evaluation Report Phosphoric acid Handling/Processing
August 4, 2021 Page 4 of 18
The reversible nature of the ionization of phosphoric acid in aqueous solution causes the formation of multiple 119
buffer systems, based on the equilibrium constants for each reaction. The buffer systems prevent dramatic 120
changes to the pH of the solution upon addition of either acid or base and are effective when the concentration of 121
weak acid and conjugate base are within ten times the other (for example, , H
3
PO
4
and H
2
PO
4
-
in Equation 1) 122
(Silberberg 2003, Kalka 2021). The specific composition of phosphoric acid is dependent on the pH of the 123
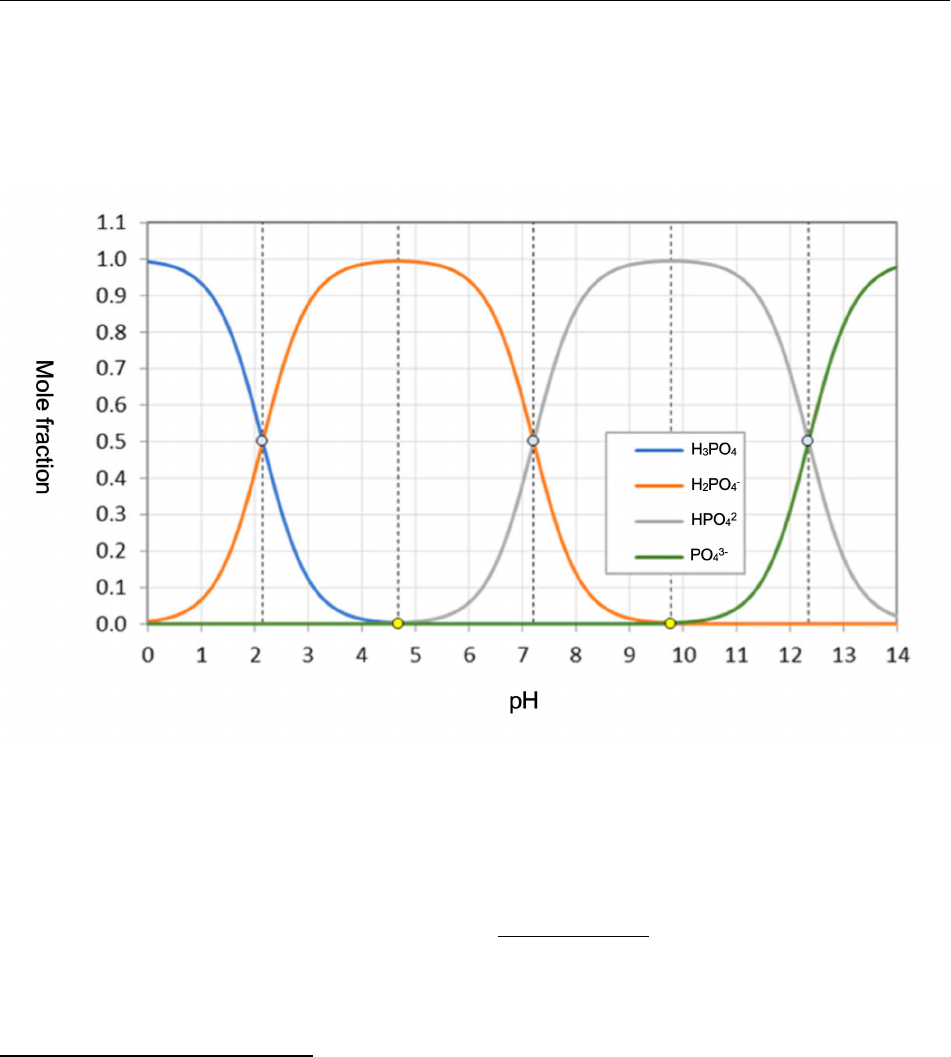
solution, as illustrated by the speciation diagram for orthophosphoric acid shown below in Figure 4 (Kalka 2021). 124
125
126
Figure 4 127
128
The pH of any of the buffer systems can be calculated by applying the Henderson-Hasselbalch equation, shown 129
below in Equation 5 (Silberberg 2003, Kalka 2021). The intersection of conjugate acid/conjugate base pairs occurs 130
when the species have equal concentrations, at which point the pH of the solution is equal to the pKa of the acid 131
(shown in Equations 2 – 4) (Silberberg 2003, Kalka 2021). 132
133
= + log
[ ]
[ ]
134
135
Equation 5 136
137
Source or Origin of the Substance: 138
Phosphoric acid is a substance that does not exist in nature but rather is produced from mineral sources in 139
the wet process or elemental phosphorous in the thermal process (EPA 1995, Shriver and Atkins 2008, 140
Gilmour 2019). The majority of wet process phosphoric acid (~85-90%) is used for the production of 141
fertilizers for conventional agriculture (Shriver and Atkins 2008). 142
143
Historically, most high-purity technical and food grade phosphoric acid is produced through the thermal 144
process to eliminate mineral impurities in the final composition (EPA 1995, Shriver and Atkins 2008). 145
However, due to the expensive nature of the thermal process, there has been continued development of 146
purification methods for wet process phosphoric acid (Shlewitt and Alibrahim 2008, Gilmour 2019). The 147
advances in phosphoric acid purification methods have made wet process the predominant method for the 148
production of technical and food grade phosphoric acid (Shlewitt and Alibrahim 2008, Jin et al.. 2014, 149
Gilmour 2019, Haghani and Daneshpazhuh 2020). 150
151
Technical Evaluation Report Phosphoric acid Handling/Processing
August 4, 2021 Page 5 of 18
152
Properties of the Substance: 153
All forms of phosphoric acid are weak polyprotic inorganic acids (Shriver and Atkins 2008, Gilmour 2019). 154
As discussed above in the “Composition of the Substance” section, phosphoric acids are capable of forming 155
buffered solutions, and may exist as a mixture of phosphoric acid and phosphate species. General 156
properties for common phosphoric acids are described below in Table 1. 157
158
Table 1. Properties of phosphoric acids 159
160
Property
Orthophosphoric
acid
Pyrophosphoric
acid
Triphosphoric
acid
Metaphosphoric
acid
Polyphosphoric
acid
Chemical formula
H
3
PO
4
H
4
P
2
O
7
H
5
P
3
O
10
H
3
PO
4
H
n+2
P
n
O
3n+1
CAS No.
7664-38-2
2466-09-3
10380-08-2
37267-86-0
8017-16-1
Molecular weight
79.97 g/mol
177.98 g/mol
257.96 g/mol
79.97 g/mol
N/A
Appearance
Clear liquid, solid
Colorless solid
Solid
Solid chips
Liquid
Water solubility
98 g/L at 20 °C
No data listed
No data listed
Melting point
40 – 42.4 °C
61 – 63 °C
Boiling point
158 °C
No data listed
Relative density
1.685 g/cm
3
No data listed
Sources: PC 983, PC 1004, PC 1023, PC 3084658, SA 2020a, SA 2020b, SA 2020c, SA 2021. 161
162
Specific Uses of the Substance: 163
Phosphoric acid is used in organic handling and processing as a cleaning agent for “food contact surfaces 164
and equipment,” as described in 7 CFR 205.605. Phosphoric acid has been approved for pH adjustment of 165
some soil amendments and as an equipment cleaner in both organic crop and livestock production. (7 CFR 166
205.601 and §205.603). 167
168
In addition to its appearance in 7 CFR 205.605, phosphoric acid has been used as an ingredient in plant 169
extractions, as described above in “Summary of Petitioned Use” (USDA 2002, USDA 2019, USDA 2020a, 170
USDA 2020b). When used in this manner, phosphoric acid acts as an acidifying agent and stabilizer to 171
facilitate more efficient extraction of target compounds (Yoon et al. 2020). 172
173
In addition to organic applications, phosphoric acid is a widely-used substance in conventional agriculture, 174
with approximately 90% of wet process phosphoric acid used in the production of fertilizers (Shriver and 175
Atkins 2008). Phosphoric acid has uses in food and beverage processing as a pH adjuster, flavor ingredient, 176
and processing agent in dairy products (Wolke 2002, Gilmour 2019). Phosphoric acid is also a precursor to 177
synthetic phosphates, which have a variety of uses including as fertilizers, surfactants, and detergents 178
(Shriver and Atkins 2008). 179
180
Approved Legal Uses of the Substance: 181
Phosphoric is listed in the USDA organic regulations, with approved uses for crop and livestock and 182
processing applications in 7 CFR 205. Phosphoric acid is listed as a “nonagricultural (nonorganic) 183
substance allowed as [an] ingredient in or on processed products labeled as “organic” or “made with 184
organic,”” and may be used for the “cleaning of food-contact surfaces and equipment only” in 7 CFR 185
205.605. Phosphoric acid is listed as a “synthetic substance allowed for use in organic livestock production 186
as an equipment cleaner, provided that no direct contact with organically managed livestock or land 187
occurs” in 7 CFR 205.603. 188
189
Phosphoric acid is listed as a “synthetic substance allowed for use in organic crop production” as a pH 190
adjustment for soil amendments in 7 CFR 205.601. Specifically, phosphoric acid can be used to adjust the 191
pH of “liquid fish products,” and “squid byproducts–from food waste processing only,” with the 192
stipulation that “the amount of acid used shall not exceed the minimum needed to lower the pH to 3.5.” 193
194
The USDA has listed phosphoric acid as an “antioxidant synergist” for the “processing and packaging [of] 195
butter and related products” in 7 CFR 58.305. 196
197

Technical Evaluation Report Phosphoric acid Handling/Processing
August 4, 2021 Page 6 of 18
The United States Food and Drug Administration (FDA) has designated phosphoric acid to be generally 198
recognized as safe (GRAS) for several uses. Phosphoric acid is listed as a “multiple purpose GRAS food 199
substance” in 21 CFR 182.1073, and as a GRAS “general purpose food additive” in §582.1073. Additionally, 200
the FDA lists phosphoric acid as a substance used in the production of the GRAS substances monobasic 201
ammonium phosphate in §184.1141, dibasic ammonium phosphate in §184.1141, magnesium phosphate in 202
§184.1366, and hydrogen peroxide in §184.1366. 203
204
The FDA has approved phosphoric acid as a component for the production of the food polymer 205
polydextrose in 21 CFR 172.841. 206
207
The FDA has approved the use of phosphoric acid as an acidifying agent in dairy products, including: 208
209
• acidified milk in §131.111 210
• cold pack-cheese and club cheese “in such quantity that the pH of the finished cold-pack cheese is 211
not below 4.5” in §133.123 and §133.124 212
• dry curd cottage cheese to facilitate curd formation in cottage cheese and “reach a pH of between 213
4.5 and 4.7” in §133.129 214
• pasteurized process cheese “in such quantity that the pH of the pasteurized process cheese is not 215
below 5.3” in §133.169 216
• pasteurized process cheese food “in such quantity that the pH of the pasteurized process cheese is 217
not below 5.0” in §133.173 218
• pasteurized Neufchatel cheese spread with other foods in §133.178 219
• pasteurized process cheese spread “in such quantity that the pH of the pasteurized process cheese 220
is not below 4.0” in §133.179 221
222
The FDA has approved the use of phosphoric acid as a neutralizing agent in cacao products, including: 223
224
• cacao nibs, with the stipulation that “for each 100 parts by weight of cacao nibs, used as such, or 225
before shelling from the cacao beans, the total quantity of phosphoric acid used is not greater than 226
0.5 part by weight, expressed as P
2
O
5
” in §163.110 227
• chocolate liquor in §163.111 228
• breakfast cacao in §163.112 229
230
The FDA has approved the use of phosphoric acid in the formulation of “color additives exempt from 231
certification,” including: 232
233
• caramel “to assist caramelization, in amounts consistent with good manufacturing practice” in 234
§73.85 235
• silver “prepared by the reaction of silver nitrate with ferrous sulfate in the presence of phosphoric 236
acid” in §73.2500 237
• manganese violet “obtained by reacting phosphoric acid, ammonium dihydrogen orthophosphate, 238
and manganese dioxide at temperatures above 450 °F” in §73.2775 239
240
The FDA has approved phosphoric acid as a component of sanitizing solutions in 21 CFR 178.1010. 241
Phosphoric acid triesters with ethylene glycol have been approved as an “antioxidant and/or stabilizer for 242
polymers” in §178.2010. Phosphoric acid has been approved as a reactant in the production of industrial 243
starch-modified articles “for use in producing, manufacturing, packing, processing, preparing, treating, 244
packaging, transporting, or holding food” in 21 CFR 178.3520. Phosphoric acid has been approved as a 245
“miscellaneous material” of resinous and polymeric coatings in 21 CFR 175.300. Phosphoric acid is 246
approved for use as an adjuvant in resin-bonded filters in 21 CFR 177.2260. 247
248
The FDA has approved phosphoric acid as a component of treatment rinses in anticaries active ingredients 249
and anticaries drug products in 21 CFR 355.10 and §310.545. 250
251
Technical Evaluation Report Phosphoric acid Handling/Processing
August 4, 2021 Page 7 of 18
The FDA has approved the use of phosphoric acid for the production of food additives for animal feeds, 252
including: 253
254
• for the hydrolysis of meat byproduct in the production of “condensed animal protein hydrolysate,” 255
in §573.200 256
• for the production of diammonium phosphate when neutralized with ammonia in §573.320 257
• as a “free-choice feed” ingredient in fenbendazole when included as “phosphoric acid 75% (feed 258
grade)” up to 2.00 percent in §558.258. 259
• as a “ruminant free-choice liquid Type C feed” ingredient in lasalocid when included as 260
“phosphoric acid (54%)” up to 3.0 percent in §558.311. 261
262
The United States Environmental Protection Agency (EPA) has identified phosphoric acid as an “inert 263
ingredient used pre- and post-harvest [with an] exemption from the requirement of a tolerance” when used 264
as a buffer in 21 CFR 180.910. The EPA has identified phosphoric acid as an ingredient “in an antimicrobial 265
pesticide formulation that may be applied to dairy processing equipment and food-processing equipment 266
and utensils” without limitation in 40 CFR 180.940. 267
268
The EPA has designated phosphoric acid and orthophosphoric acid as a hazardous substance in 40 CFR 269
116.4 and §302.4, with a final reportable quantity of 5000 pounds or 2270 kg. The EPA has listed wastes 270
generated from process wastewater and phosphogypsum from phosphoric acid production as “solid 271
waste” in 40 CFR 261.4 272
273
The EPA limits the pollutant content of process and non-process wastewater from phosphoric acid 274
production to the levels shown in Table 2 below, as stipulated in 40 CFR 418.12, §418.13, §418.15, §422.52, 275
§422.53, and §422.55 276
277
Table 2. Effluent limitations for wastewater from phosphoric acid production 278
279
Effluent characteristic Maximum for any 1 day
Average of daily values for 30
consecutive days shall not exceed—
Total phosphorus (as P)
105
35
Fluoride
75
25
TSS [total suspended solids]
150 (process wastewater only)
50 (process wastewater only)
pH
6.0 to 9.5
6.0 to 9.5
280
The United States Occupational Safety and Health Administration (OSHA) has listed phosphoric acid as an 281
air contaminant with a maximum concentration of 1 mg/m
3
in 29 CFR 1910.1000. 282
283
Action of the Substance: 284
When used for plant extractions, phosphoric acid facilitates the extraction of target molecules by lowering 285
the pH of solution and stabilizing target molecules against decomposition. The acidic nature of phosphoric 286
acid results in its ability to lower the pH of solutions used for extractions. By changing the pH of the 287
extraction solution, the solubility of acidic and basic compounds can be manipulated to improve their 288
solubility in the extraction solvent (Pavia et al. 1995). The acidic pH produced by the addition of 289
phosphoric acid to extraction mixtures will result in the protonation of basic functional groups (e.g., 290
amines), increasing the net charge of target molecules and increasing their solubility in polar solvents 291
(Pavia et al. 1995, Silberberg 2003, Albuquerque et al. 2005, Nicoué et al. 2007, Dai and Mumper 2010, 292
Timberlake 2016). 293
294
The ability to protonate functional groups is dependent on the strength of the specific acid, described by 295
the acid pKa value (lower pKa = stronger acid) (Silberberg 2003, Shriver and Atkins 2008, Kalka 2021). The 296
relatively high strength of phosphoric acid (pKa
1
= 2.15) allows for an environment that is sufficiently 297
acidic to ensure that carboxylic acid groups (pKa ≈ 5) remain protonated and neutrally charged (Silberberg 298
2003, Timberlake 2016). The manipulation of the molecular charge of target compounds enhances their 299
extraction from solid or liquified plant material (Porter and Lodge 2021). Acidic solutions also improve the 300
Technical Evaluation Report Phosphoric acid Handling/Processing
August 4, 2021 Page 8 of 18
extraction of some molecules as the acid is able to degrade cell walls, lignin, cellulose and other structural 301
components, improving the accessibility of target molecules. (Revilla et al. 1998, Albuquerque et al. 2005, 302
Dai and Mumper 2010, Zeng et al. 2014, Yao et al. 2017). 303
304
In addition to changing the charge and solubility profile for target compounds, phosphoric acid also acts as 305
a stabilizer in extraction processes. The stabilizing nature of phosphoric acid is possible through two main 306
mechanisms. One mechanism is connected to the manipulation of charge and solubility as discussed in the 307
paragraph above. Some compounds are less susceptible to decomposition in charged states (Revilla et al. 308
1998, Nicoué et al. 2007, Dai and Mumper 2010, Porter and Lodge 2021). The improved stability of some 309
salts in comparison to their related neutral compounds is commonly used to protect amines and other 310
sensitive compounds from undesired reactions (Albuquerque et al. 2005, Nicoué et al. 2007). Additionally, 311
the acidic pH established by phosphoric acid may denature plant proteins and oxidizing enzymes, 312
preventing the oxidation of antioxidants and other sensitive target compounds (Nicoué et al. 2007, Dai and 313
Mumper 2010, Timberlake 2016, Porter and Lodge 2021). 314
315
Combinations of the Substance: 316
When used as an ingredient for plant extractions, phosphoric acid is combined with the extraction solvent. 317
The solvent varies dependent on the plant and target molecule, but common extraction solvents include 318
water, alcohols (e.g., methanol, ethanol, isopropanol, etc.), and ketones (e.g., acetone) (Nicoué et al. 2007, 319
Dai and Mumper 2010, Yoon et al. 2020). 320
321
Status 322
323
Historic Use: 324
Phosphoric acid has been historically used in organic agriculture production as a cleaner and pH adjuster. 325
Phosphoric acid has been historically used in fertilizer and animal feed production within conventional 326
agriculture. Fertilizer production continues to be the most prominent application of phosphoric acid 327
(Shriver and Atkins 2008, Gilmour 2019). Additionally, phosphoric acid has been used as a pH adjuster and 328
flavoring ingredient in food and beverage production, and as an industrial cleaner and source of phosphate 329
detergents in many industries, including textiles, laundry, and dishwasher applications (Flomenbaum et al. 330
2002, Wolke 2002, Shriver and Atkins 2008). 331
332
Organic Foods Production Act, USDA Final Rule: 333
Phosphoric acid is not listed in the Organic Foods Production Act of 1990 (OFPA). However, phosphoric 334
acid is listed in the USDA organic regulations, with approved uses for crop and livestock and processing 335
applications in 7 CFR Part 205. Phosphoric acid may be used for the “cleaning of food-contact surfaces and 336
equipment only” in 7 CFR 205.605. Phosphoric acid can be used to adjust the pH of “liquid fish products,” 337
and “squid byproducts–from food waste processing only,” with the stipulation that “the amount of acid 338
used shall not exceed the minimum needed to lower the pH to 3.5” in 7 CFR 205.601. Phosphoric acid is 339
listed as a “synthetic substance allowed for use in organic livestock production as an equipment cleaner, 340
provided that no direct contact with organically managed livestock or land occurs” in 7 CFR 205.603. 341
342
International 343
344
Canada, Canadian General Standards Board—CAN/CGSB-32.311-2015, Organic Production Systems 345
Permitted Substances List 346
Phosphoric acid is listed in the Organic Production Systems Permitted Substances List as an approved 347
substance for pH adjustment of “fish meal, fish powder, fish wastes, hydrolysate, emulsions and solubles” 348
that are used for “soil amendments and crop nutrition.” Phosphoric acid is also listed as a “cleaner, 349
disinfectant and sanitizer permitted on organic product contact surfaces for which a removal event is 350
mandatory [for use] on dairy equipment.” 351
352
CODEX Alimentarius Commission—Guidelines for the Production, Processing, Labelling and 353
Marketing of Organically Produced Foods (GL 32-1999) 354
Phosphoric acid is not listed in the CODEX. 355

Technical Evaluation Report Phosphoric acid Handling/Processing
August 4, 2021 Page 9 of 18
356
European Economic Community (EEC) Council Regulation—EC No. 834/2007 and 889/2008 357
Phosphoric acid is not listed in EC No. 834/2007 or EC No. 889/2008. 358
359
Japan Agricultural Standard (JAS) for Organic Production 360
Phosphoric acid is not listed in the JAS. 361
362
363
International Federation of Organic Agriculture Movements (IFOAM) 364
Phosphoric acid is listed in the IFOAM NORMS for organic production and processing as an “equipment 365
cleanser and equipment disinfectant only for dairy equipment,” and as a “substance for pest and disease 366
control and disinfection in livestock housing and equipment [for] dairy equipment.” 367
368
Evaluation Questions for Substances to be used in Organic Handling 369
370
Evaluation Question #1: Describe the most prevalent processes used to manufacture or formulate the 371
petitioned substance. Further, describe any chemical change that may occur during manufacture or 372
formulation of the petitioned substance when this substance is extracted from naturally occurring plant, 373
animal, or mineral sources (7 U.S.C. § 6502 (21)). 374
375
Phosphoric acid is produced through two methods, the wet process and the thermal process (EPA 1995, 376
Gilmour 2019, Haghani and Daneshpazhuh 2020). Historically, the end-point use for the phosphoric acid 377
was determined by its production method. High purity, technical and food grade phosphoric acid was 378
produced by the thermal process (EPA 1995, Gilmour 2019). Lower purity phosphoric acid, primarily used 379
in animal feed and fertilizer applications, was produced by the wet process (EPA 1995, Shriver and Atkins 380
2008, Gilmour 2019). Due to the expensive nature of the thermal process, there has been continued 381
development of purification methods for wet process phosphoric acid, which now serve as the 382
predominant method for the production of technical and food grade phosphoric acid (Gilmour 2019). 383
384
Thermal process 385
386
The thermal process is broken down into three major steps: combustion, hydration, and demisting 387
(collection) (EPA 1995, Gilmour 2019). In the combustion step, elemental yellow phosphorus (P
4
) is reacted 388
with oxygen gas, which oxidizes the phosphorous from its 0 to V oxidation state, as shown below in 389
Equation 6 (EPA 1995, Gilmour 2019). The heat of combustion for phosphorus is highly endothermic and 390
the reaction must be carried out at high temperatures (1650 – 2760 °C) (EPA 1995, Gilmour 2019). 391
392
P
4
+ 5 O
2
2 P
2
O
5
393
394
Equation 6 395
396
Once the elemental phosphorus is oxidized to P
2
O
5
, it undergoes the hydration process to form 397
orthophosphoric acid, as shown below in Equation 7 (EPA 1995, Gilmour 2019). In this process P
2
O
5
is 398
generally reacted with water, although in some cases dilute solutions of phosphoric acid are used instead 399
of water alone (EPA 1995). Once phosphoric acid has been produced, it is isolated in the demisting process. 400
In this step, phosphoric acid is collected as a mist with high-pressure drop demisters. The thermal process 401
produces phosphoric acid with P
2
O
5
concentrations between 54 and 62%, which are sufficiently pure for 402
use in technical and food grade applications (EPA 1995, Gilmour 2019). 403
404
2 P
2
O
5
+ 6 H
2
O
4 H
3
PO
4
405
406
Equation 7 407
408
Wet Process 409
410
Technical Evaluation Report Phosphoric acid Handling/Processing
August 4, 2021 Page 10 of 18
The wet process produces phosphoric acid from naturally occurring phosphate mineral sources 411
(fluorapatite [Ca
10
(PO
4
)
6
F
2
] and hydroxyapatite [Ca
10
(PO
4
)
6
(OH)
2
]) (EPA 1995, Shriver and Atkins 2008, 412
Gilmour 2019, Haghani and Daneshpazhuh 2020). Once mined, these minerals are converted to phosphoric 413
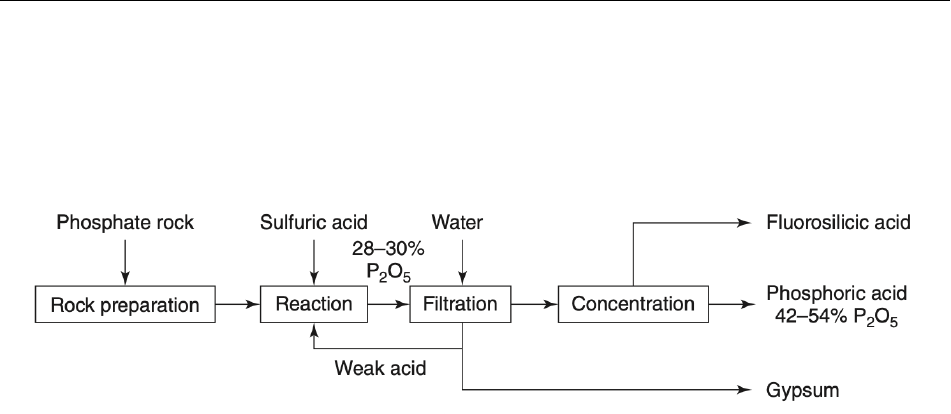
acid in four main steps, as outlined in Figure 5 below (Gilmour 2019). The phosphate rock is prepped in the 414
initial step by being milled and ground to increase its surface area (EPA 1995, Haghani and Daneshpazhuh 415
2020). 416
417
418
419
Figure 5 420
421
Once milled, the mineral phosphates are reacted with a strong mineral acid and converted to phosphoric 422
acid, as shown in Equation 8 below (EPA 1995, Shriver and Atkins 2008, Gilmour 2019, Haghani and 423
Daneshpazhuh 2020). While sulfuric acid is shown in both Figure 5 and Equation 8, other strong mineral 424
acids (e.g., nitric acid [HNO
3
] and hydrochloric acid [HCl]) may also be used (Jin et al. 2014, Haghani and 425
Daneshpazhuh 2020). However, most commercial processes use sulfuric acid because it provides higher 426
phosphoric acid yields, lower costs, and a solid form of calcium (Al-Fariss et al. 1992, EPA 1995, Shriver 427
and Atkins 2008, Gilmour 2019). The specific reaction conditions dictate the type of calcium sulfate hydrate 428
(CaSO
4
• n H
2
O) formed, with lower temperatures favoring the formation of gypsum (CaSO
4
• 2 H
2
O), as 429
shown in Equation 8 (EPA 1995). The prevalence of fluorapatite among mineral phosphates also produces 430
hydrofluoric acid (HF), as shown below in Equation 8. 431
432
Ca
10
(PO
4
)
4
F
2 (s)
+ H
2
SO
4 (aq)
+ 20 H
2
O
(l)
6 H
3
PO
4 (aq)
+ 10 [CaSO
4
• 2 H
2
O]
(s)
+ 2 HF
(aq)
433
434
Equation 8 435
436
The gypsum formed during the reaction with the mineral acid is removed via filtration. Once removed, the 437
gypsum solids undergo several aqueous wash cycles to remove residual phosphoric acid from the solid 438
surface, producing phosphoric acids yields of 99.9% (EPA 1995, Gilmour 2019). As shown previously in 439
Figure 5, the aqueous gypsum washes are sent back to the reaction vessel to aid in the conversion of 440
mineral phosphates (EPA 1995, Gilmour 2019). The presence of mineral silicon in the initial composition 441
reacts with hydrofluoric acid to produce less reactive forms of silicon tetrafluoride (SiF
4
) and SiF
6
2-
ions, 442
some of which are removed as solids with the gypsum (Gilmour 2019). 443
444
The phosphoric acid isolated following the filtration process is dilute, with P
2
O
5
concentrations between 26 445
– 30% (EPA 1995, Gilmour 2019). Vacuum evaporation is used to remove water and concentrate the 446
phosphoric acid to 42 – 54% P
2
O
5
(Gilmour 2019). Activated silica or clay is added during the concentration 447
process to react with residual hydrofluoric acid. Silicon tetrafluoride isolated from the concentration step is 448
hydrolyzed to fluorosilicic acid (H
2
SiF
6
), as shown in Figure 5 (Gilmour 2019). 449
450
Mineral impurities, including heavy metal contaminants, remain in phosphoric acid produced via the wet 451
process, which have historically limited its use to agricultural fertilizer applications (EPA 1995, Shriver and 452
Atkins 2008, Gilmour 2019, Haghani and Daneshpazhuh 2020). Wet process phosphoric acid results in 453
concentrations of between 42 and 54% P
2
O
5
, which is largely unsuitable for technical applications (Gilmour 454
2019). The elemental phosphorous used in the thermal process can be purified via sublimation, resulting in 455
no carry-over of heavy metal contaminants so that thermal phosphoric acid can be used in technical and 456

Technical Evaluation Report Phosphoric acid Handling/Processing
August 4, 2021 Page 11 of 18
food applications (Shriver and Atkins 2008). However, the thermal process is much more expensive and 457
energy intensive than the wet process (~2000 °C vs ~80 °C) (EPA 1995, Gilmour 2019). 458
459
Wet process purification methods 460
461
Wet process phosphoric acid is commonly purified by crystallization or solvent extraction (Gilmour 2019). 462
Crystallization is a common purification technique, which is based on the differing solubilities of pure and 463
impure mixtures, with pure substances selectively crystallizing at reduced temperatures (Pavia et al. 1995). 464
When phosphoric acid is concentrated to 61% P
2
O
5
or higher, it selectively forms hemihydrate crystals 465
(H
3
PO
4
• ½ H
2
O) when cooled to 8 – 12 °C (Gilmour 2019). The crystals are removed from the mixture and 466
can be melted to undergo additional recrystallization cycles to improve purity, with each cycle yielding a 467
10 to 100 times increase in purity (Gilmour 2019). 468
469
Solvent extraction is another traditional purification method based on solubility. In solvent extraction, the 470
target compound migrates between immiscible phases (usually aqueous [polar] and organic [nonpolar]) 471
based on solubility (Pavia et al. 1995). The selectivity of phosphoric acid does not differ greatly compared 472
to its impurities, requiring additional purification steps. Prior to solvent extraction, concentrated 473
phosphoric acid undergoes precipitation with calcium or barium salts to remove sulfate (SO
4
2-
), sodium 474
salts to remove fluorosilicates, and sulfides to remove arsenic (Shlewitt and Alibrahim 2008, Gilmour 2019, 475
Haghani and Daneshpazhuh 2020). Phosphoric acid extractions are performed in one or more extraction 476
columns with many possible organic solvents, including alcohols, ethers, ketones, amines, and kerosene 477
blends (Shlewitt and Alibrahim 2008, Jin et al. 2014, Gilmour 2019). Following extraction with an organic 478
solvent, phosphoric acid is recovered with water. Residual organic solvents are removed via evaporation 479
during the concentration of the recovered phosphoric acid from the aqueous solution (Shlewitt and 480
Alibrahim 2008, Gilmour 2019). Solvent extraction of wet process phosphoric acid improves the purity of 481
the substance from 42-54% P
2
O
5
in the raw form to up to 97% P
2
O
5
(Gilmour 2019). 482
483
Evaluation Question #2: Discuss whether the petitioned substance is formulated or manufactured by a 484
chemical process, or created by naturally occurring biological processes (7 U.S.C. § 6502 (21)). Discuss 485
whether the petitioned substance is derived from an agricultural source. 486
487
Phosphoric acid is not a naturally occurring substance. As described in Evaluation Question 1, phosphoric 488
acid can be derived from natural phosphate minerals in the wet process or elemental phosphorus in the 489
thermal process. In both methods, phosphoric acid is produced through chemical processes. 490
491
According to the NOP decision trees, phosphoric acid is classified as a nonagricultural, synthetic substance 492
due to its chemical change from a natural mineral phosphate to an acid during processing (NOP 2016a, 493
NOP 2016b). Furthermore, the mineral source of phosphoric acid is classified as a nonagricultural source 494
(NOP 2016a, NOP 2016b). 495
496
Evaluation Question #3: If the substance is a synthetic substance, provide a list of nonsynthetic or 497
natural source(s) of the petitioned substance (7 CFR 205.600(b)(1)). 498
499
As described in Evaluation Questions 1 – 2, phosphoric acid is a synthetic substance that does not exist in 500
nature. Therefore, there are no natural sources of phosphoric acid. 501
502
Evaluation Question #4: Specify whether the petitioned substance is categorized as generally 503
recognized as safe (GRAS) when used according to FDA’s good manufacturing practices (7 CFR 504
205.600(b)(5)). If not categorized as GRAS, describe the regulatory status. 505
506
As described in the “Approved Legal Uses of the Substance” section, the FDA has designated phosphoric 507
acid generally recognized as safe (GRAS) for several uses. Phosphoric acid is listed as a “multiple purpose 508
GRAS food substance” in 21 CFR 182. 1073, and as a GRAS “general purpose food additive” in §582.1073. 509
Additionally, the FDA lists phosphoric acid as a substance used in the production of the GRAS substances 510

Technical Evaluation Report Phosphoric acid Handling/Processing
August 4, 2021 Page 12 of 18
monobasic ammonium phosphate in §184.1141, dibasic ammonium phosphate in §184.1141, magnesium 511
phosphate in §184.1366, and hydrogen peroxide in §184.1366. 512
513
Evaluation Question #5: Describe whether the primary technical function or purpose of the petitioned 514
substance is a preservative. If so, provide a detailed description of its mechanism as a preservative 515
(7 CFR 205.600(b)(4)). 516
517
When used as petitioned, the primary function of phosphoric acid is to improve the extraction of target 518
molecules, not to act as a preservative. However, in some cases, the addition of phosphoric acid stabilizes 519
target molecules from decomposition, as described above in the “Action of the Substance” section. 520
521
Phosphoric acid is also used as an equipment sanitizer in organic agriculture in 7 CFR 205.605 and 522
§205.603. The low pH of phosphoric acid solutions makes it an antimicrobial substance, as high acid 523
content is not tolerated by microorganisms (Winniczuk and Parish 1997, Prado et al. 2015). The 524
antimicrobial nature of phosphoric acid may result in some preservative characteristics if incorporated into 525
food and beverage products (Winniczuk and Parish 1997). 526
527
Evaluation Question #6: Describe whether the petitioned substance will be used primarily to recreate 528
or improve flavors, colors, textures, or nutritive values lost in processing (except when required by law) 529
and how the substance recreates or improves any of these food/feed characteristics (7 CFR 205.600(b)(4)). 530
531
When used as petitioned, the primary function of phosphoric acid is to improve the extraction of target 532
molecules, not to improve or recreate flavors in processed food products. However, phosphoric acid has 533
been used as a flavoring agent in conventional food and beverage production, as described above in the 534
“Specific Uses of the Substance” and “Historical Use” sections. 535
536
Evaluation Question #7: Describe any effect or potential effect on the nutritional quality of the food or 537
feed when the petitioned substance is used (7 CFR 205.600(b)(3)). 538
539
When used as petitioned, phosphoric acid will be used in the extraction of target molecules from plant 540
material. The extraction of antioxidants and other compounds from the initial plant material will reduce 541
the nutritional quality of the material from which they are extracted. However, the purpose of plant 542
extracts is to improve the quality of other products to which they are added. (Nicoué et al. 2007, Proestos 543
2020). Phosphoric acid is a source of phosphates, which are important nutrients in human health, and can 544
be found in many biomolecules, including ATP and DNA (Shriver and Atkins 2008, Timberlake 2016, 545
Gilmour 2019). However, phosphoric acid is typically used in low concentrations (1 – 3%) in extraction 546
processes and is unlikely to contribute directly to improved nutritional quality. 547
548
Evaluation Question #8: List any reported residues of heavy metals or other contaminants in excess of 549
FDA tolerances that are present or have been reported in the petitioned substance (7 CFR 205.600(b)(5)). 550
551
As described in Evaluation Questions 1 – 2, wet process phosphoric acid is produced from mineral 552
phosphates. The presence of heavy metals in the initial mineral source may result in carry over to the 553
phosphoric acid product (Haghani and Daneshpazhuh 2020). The prevalence of contaminants in 554
phosphoric acids based on their source and application are listed below in Table 3 (Gilmour 2019). 555
556
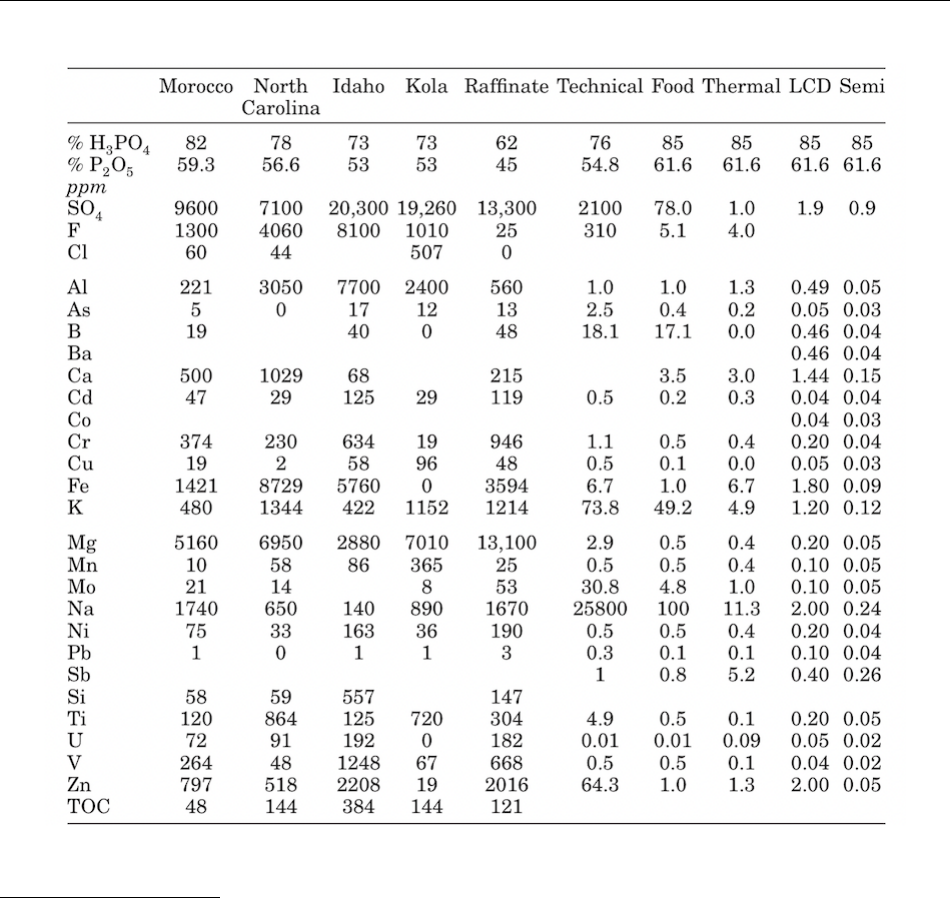
Technical Evaluation Report Phosphoric acid Handling/Processing
August 4, 2021 Page 13 of 18
Table 3. Impurities in phosphoric acid 557
558
559
Source: Gilmour 2019. 560
561
Evaluation Question #9: Discuss and summarize findings on whether the manufacture and use of the 562
petitioned substance may be harmful to the environment or biodiversity (7 U.S.C. § 6517 (c) (1) (A) (i) 563
and 7 U.S.C. § 6517 (c) (2) (A) (i)). 564
565
As described in Evaluation Question 7, when used as petitioned phosphoric acid is used in low 566
concentrations (1 – 3%), and is a source of phosphates for incorporation to biomolecules. The low 567
concentration in extraction applications and the prevalence of phosphates throughout biology make 568
phosphoric acid from plant extractions unlikely to be harmful to the environment or biodiversity. 569
570
However, the production of phosphoric acid does have the potential to be harmful to the environment. As 571
described in Evaluation Question 1, the thermal process for producing phosphoric acid is energy intensive 572
and requires high temperatures. The high energy requirements of the thermal process may contribute to 573
atmospheric CO
2
levels if the energy is produced from fossil fuels. The thermal process also requires the 574
treatment of combustion gases by scrubbers, cyclonic separators, mist eliminators, and electrostatic 575
precipitators to prevent the release of phosphoric acid to the environment (EPA 1995, Gilmour 2019). The 576
small size (< 3 m diameter) makes these phosphoric acid and phosphorus oxide (P
2
O
5
) particles difficult 577
to capture, and contributes their release to the atmosphere at levels of “< 25 mg P
2
O
5
per dry standard 578
cubic meter of stack gas” (Gilmour 2019). 579
580
Wet process phosphoric acid is produced from chemical changes to mined mineral phosphates. There may 581
be initial harm to the environment and biodiversity in the mining process. Once the minerals are isolated, 582

Technical Evaluation Report Phosphoric acid Handling/Processing
August 4, 2021 Page 14 of 18
hydrofluoric acid presents the most likely source of environmental harm (Shriver and Atkins 2008). As 583
described in Evaluation Question 1, hydrofluoric acid is removed as a solid or as fluorosilicic acid by 584
reaction with silica sources. These include natural silicates present within the initial mineral, as well as 585
activated silica and clay added during the manufacturing process (Shriver and Atkins 2008, Gilmour 2019). 586
Additionally, scrubbers are used to remove gaseous fluorine compounds from concentration steps to 587
prevent their release to the environment (EPA 1995). 588
589
In addition to the hazards from fluorine compounds, the gypsum produced may pose a hazard to the 590
environment. Isolated gypsum may be used for other commercial applications if it is sufficiently pure 591
(Gilmour 2019). In other cases, gypsum is left in gypsum stacks, or pumped out to sea (Gilmour 2019). 592
However, the gypsum may also contain silicon fluorides, acids, and other impurities from the initial 593
mineral source, which has resulted in its designation as a hazardous substance by the EPA in 40 CFR 261.4. 594
595
Evaluation Question #10: Describe and summarize any reported effects upon human health from use of 596
the petitioned substance (7 U.S.C. § 6517(c)(1)(A)(i), 7 U.S.C. § 6517(c)(2)(A)(i)) and 7 U.S.C. § 6518(m)(4)). 597
598
Concentrated phosphoric acid is corrosive, and can result in burning and irritation of the eyes and skin on 599
contact (Flomenbaum et al. 2002, NJDHSS 2004, Gilmour 2019). Phosphoric acid can desiccate epithelial 600
cells, resulting in the drying and cracking of skin where long-term exposure occurs (Flomenbaum et al. 601
2002, NJDHSS 2004). Inhalation of phosphoric acid may result in irritation to the nose, lungs, and throat 602
and may induce coughing and wheezing (NJDHSS 2004, Gilmour 2019). Ingestion of phosphoric acid may 603
damage gastric and esophageal mucus linings (Flomenbaum et al. 2002). 604
605
Phosphoric acid is frequently used in food processing and production and is a common component of food 606
and beverages (Wolke 2002). As described in Equations 2 – 4 in the “Composition of the Substance” section, 607
phosphoric acid is the source of several phosphates, which are important components of biomolecules (e.g., 608
ATP, DNA, etc.) (Shriver and Atkins 2008, Timberlake 2016, Gilmour 2019). When used as petitioned, 609
phosphoric acid is used in low concentrations (1 – 3%), making it unlikely to be harmful to human health 610
(Gilmour 2019). 611
612
Evaluation Question #11: Describe any alternative practices that would make the use of the petitioned 613
substance unnecessary (7 U.S.C. § 6518(m)(6)). 614
615
There are alternative methods to extract target molecules from plant material. One of the simplest ways to 616
improve solvent extraction processes is to increase the solvent temperature (Pavia et al. 1995, Silberberg 617
2003). Increased temperature improves the solvation of most solids and liquids by disrupting the 618
intermolecular forces that prevent the target molecule from entering the solution (Silberberg 2003). 619
620
Supercritical carbon dioxide extraction offers an alternative to acidic extractions. This extraction method 621
uses temperatures and pressures that push the solvent beyond its critical point, so that it no longer exists as 622
a liquid or gas (Silberberg 2003, Babovic et al. 2010). Carbon dioxide is the most common supercritical fluid 623
used in extraction applications due to its low cost and the low temperatures and pressures required to 624
reach supercritical conditions (31.1 °C and 7.38 MPa) (Babovic et al. 2010). The selectivity of supercritical 625
fluids can be modulated by changing its temperature and pressure to target different classes of molecules. 626
627
Subcritical extractions offer another alternative to acidic extractions. In such applications, the solvent 628
remains in liquid form, although conditions may approach the critical point of the solvent (Ibañez et al. 629
2003). As with supercritical fluid extractions, the selectivity of the subcritical extractions can be 630
manipulated by modifying temperature and pressure. Subcritical water extractions have been successful in 631
the extraction of essential oils and antioxidants (Ibañez et al. 2003). However, some antioxidants and other 632
compounds are sensitive to decomposition, and may not survive increased solvent temperatures or the 633
high pressure conditions needed in supercritical and subcritical extractions (Ibañez et al. 2003). 634
635
Technical Evaluation Report Phosphoric acid Handling/Processing
August 4, 2021 Page 15 of 18
Evaluation Question #12: Describe all natural (non-synthetic) substances or products which may be 636
used in place of a petitioned substance (7 U.S.C. § 6517(c)(1)(A)(ii)). Provide a list of allowed substances 637
that may be used in place of the petitioned substance (7 U.S.C. § 6518(m)(6)). 638
639
Many natural and currently allowed synthetic acids offer an alternative to phosphoric acid for plant 640
extractions, such as acetic acid, citric acid, gibberellic acid, lactic acid, and tartaric acid (NOP 2016c). 641
Polyprotic carboxylic acids (for example, ascorbic acid, citric acid, etc.) are also able to chelate positively 642
charged species, facilitating improved extraction (Albuquerque et al. 2005). 643
644
However, the strength of the acid is important in determining the effectiveness in the extraction of the 645
target molecules. Carboxylic acids are weaker acids than phosphoric acid (pKa ~5 vs 2.15) meaning that 646
they may be less effective in extracting some molecules, including anthocyanin antioxidants (Silberberg 647
2003, Nicoué et al. 2007, Timberlake 2016). The target molecule and plant structure determine the optimal 648
solvent conditions, although phosphoric acid solutions have been reported to be among the most effective 649
for antioxidant extractions (Nicoué et al. 2007). 650
651
Evaluation Information #13: Provide a list of organic agricultural products that could be alternatives for 652
the petitioned substance (7 CFR 205.600(b)(1)). 653
654
Alternatives to phosphoric acid are naturally acidic agricultural substances, including wine and vinegar. 655
Both mixtures include natural acids that can provide an acidic extraction solution. However, as described 656
in Evaluation Question 12, carboxylic acids are weaker than phosphoric acid and may be less effective in 657
the extraction of some target molecules. Additionally, the complex mixture of compounds in wine and 658
vinegar would make purification of the plant extracts more difficult. 659
660
Report Authorship 661
662
The following individuals were involved in research, data collection, writing, editing, and/or final 663
approval of this report: 664
665
• Philip Shivokevich, Chemistry Lecturer, California State University Bakersfield 666
• Laura M. Weinberg, Technical Editor, Savan Group 667
668
All individuals are in compliance with Federal Acquisition Regulations (FAR) Subpart 3.11—Preventing 669
Personal Conflicts of Interest for Contractor Employees Performing Acquisition Functions. 670
671
References 672
673
Albuquerque B, Lidon FC, Leitão AE. 2005. Ascorbic acid quantification in melon samples – the importance 674
of the extraction medium for HPLC analysis. General and Applied Plant Physiology. 31(3-4): 247-675
251. 676
677
Al-Fariss TF, Özbelge HÖ, El-Shall HSH. 1992. Process technology for phosphoric acid production in Saudi 678
Arabia. Journal of King Saud University. 4(2): 239-255. 679
680
Babovic N, Djilas S, Jadranin M, Vajs V, Ivanovic J, Petrovic S, Zizovic I. 2010. Supercritical carbon dioxide 681
extraction of antioxidant fractions from selected Lamiaceae herbs and their antioxidant capacity. 682
Innovative Food Science and Emerging Technologies. 11: 98-107. 683
684
Dai J, Mumper RJ. 2010. Plant phenolics: extraction, analysis and their antioxidant and anticancer 685
properties. Molecules. 15: 7313-7352. 686
687
Technical Evaluation Report Phosphoric acid Handling/Processing
August 4, 2021 Page 16 of 18
[EPA] United States Environmental Protection Agency. 1995. Chapter 8.9: Phosphoric acid in AP-42: 688
Compilation of air emissions factors. [accessed 2021 May 8]. 689
https://www3.epa.gov/ttnchie1/ap42/ch08/final/c08s09.pdf
690
691
Flomenbaum NE, Goldfrank LR, Hoffman RS, Howland MA, Lewin NA, Nelson LS. 2002. Goldfrank’s 692
Toxicologic Emergencies. 10th ed. New York (NY): McGraw-Hill. 693
694
Gilmour RB. 2019. Phosphoric acids and phosphates in Kirk-Othmer Encyclopedia of Chemical 695
Technology. John Wiley & Sons, Inc. 696
697
Haghani M, Daneshpazhuh. 2020. A novel multi-step purification method for production of profitable food 698
grade phosphoric acid and ammonium based fertilizers from a sedimentary ore. Journal of 699
Environmental Analytical Chemistry. 7(4): 2723. 700
701
Ibañez E, Kubátová A, Señoráns FJ, Cavero S, Reglero G, Hawthorne SB. 2003. Subcritical water extraction 702
of antioxidant compounds from rosemary plants. Journal of Agricultural and Food Chemistry. 51: 703
375-382. 704
705
Jin Y, Ma Y, Weng Y, Jia X, Li J. 2014. Solvent extraction of Fe
3+
from the hydrochloric acid route 706
phosphoric acid by DP3EHPA in kerosene. Journal of Industrial and Engineering Chemistry. 20: 707
3446-3452. 708
709
Kalka H. 2021. Polyprotic acids and beyond – an algebraic approach. Chemistry. 3(2): 454-508. 710
711
[NOP] National Organic Program. 2016a. 5033-1 Guidance decision tree for classification of materials as 712
synthetic or nonsynthetic. [accessed 2021 May 17]. 713
https://www.ams.usda.gov/sites/default/files/media/NOP-Synthetic-NonSynthetic-714
DecisionTree.pdf
715
716
[NOP] National Organic Program. 2016b. 5033-2 Guidance decision tree for classification of agricultural 717
and nonagricultural materials for organic livestock production or handling. [accessed 2021 May 718
17]. https://www.ams.usda.gov/sites/default/files/media/NOP-Ag-NonAg-DecisionTree.pdf
719
720
[NOP] National Organic Program. 2016c. 5034-1 Guidance materials for organic crop production. [accessed 721
2021 May 17]. https://www.ams.usda.gov/sites/default/files/media/NOP-5034-1.pdf
722
723
[NOP] National Organic Program. 2013. Memorandum to the National Organic Standards Board. [accessed 724
2021 Mar 25]. 725
https://www.ams.usda.gov/sites/default/files/media/Phos%20acid%202002%20NOP%20memo726
%20to%20NOSB.pdf
727
728
[NJDHSS] New Jersey Department of Health and Senior Services. 2004. Phosphoric acid hazardous 729
substance fact sheet. [accessed 2021 May 17]. 730
https://nj.gov/health/eoh/rtkweb/documents/fs/1516.pdf
731
732
Nicoué EE, Savard S, Belkacemi K. 2007. Anthocyanins in wild blueberries of Quebec: extraction and 733
identification. Journal of Agricultural and Food Chemistry. 55: 5626-5635. 734
735
Pavia DL, Lampman GM, Kriz GS, Engel RG. 1995. Introduction to organic laboratory techniques: a 736
macroscale approach. 2nd ed. Orlando (FL): Harcourt Brace & Company. 737
738
Porter K, Lodge JK. 2021. Determination of selected water-soluble vitamins (thiamine, riboflavin, 739
nicotinamide and pyridoxine) from a food matrix using hydrophilic interaction liquid 740
chromatography coupled with mass spectroscopy. Journal of Chromatography B. 1171: 122541. 741
742

Technical Evaluation Report Phosphoric acid Handling/Processing
August 4, 2021 Page 17 of 18
Prado M, Silva EJNL, Duque TM, Zaia AA, Ferraz CCR, Almeida JFA, Gomes BPFA. 2015. Antimicrobial 743
and cytotoxic effects of phosphoric acid solution compared to other root canal irrigants. Journal of 744
Applied Oral Science. 23(2): 1678-7757. 745
746
Proestos C. 2020. The benefits of plant extracts for human health. Foods. 9: 1653. 747
748
[PC] PubChem Database. 2005. Metaphosphoric acid, CID = 3084658. National Center for Biotechnology 749
Information. [modified 2021 May 8, accessed 2021 May 14]. 750
https://pubchem.ncbi.nlm.nih.gov/compound/Metaphosphoric-acid
751
752
[PC] PubChem Database. 2016. Phosphoric acid, CID = 1004. National Center for Biotechnology 753
Information. [modified 2021 May 8, accessed 2021 May 14]. 754
https://pubchem.ncbi.nlm.nih.gov/compound/Phosphoric-acid
755
756
[PC] PubChem Database. 2016. Pyrophosphoric acid, CID = 1023. National Center for Biotechnology 757
Information. [modified 2021 May 8, accessed 2021 May 14]. 758
https://pubchem.ncbi.nlm.nih.gov/compound/Pyrophosphoric-acid
759
760
[PC] PubChem Database. 2005. Triphosphoric acid, CID = 983. National Center for Biotechnology 761
Information. [modified 2021 May 8, accessed 2021 May 14]. 762
https://pubchem.ncbi.nlm.nih.gov/compound/Triphosphoric-acid
763
764
Revilla E, Ryan JM, Martin-Ortega G. 1998. Comparison for several procedures used for the extraction of 765
anthocyanins from red grapes. Journal of Agricultural and Food Chemistry. 46: 4592-4597. 766
767
Shlewitt H, Alibrahim M. 2008. Counter current extraction of phosphoric acid: food grade acid production. 768
Chemical Engineering. 52(1): 7-9. 769
770
Shriver DF, Atkins PW. 2008. Inorganic Chemistry. 4th ed. New York (NY): W.H. Freeman and Company. 771
772
[SA] Sigma-Aldrich Inc. 2020a. Metaphosphoric acid sds [accessed 2021 May 7 2021]. 773
https://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en774
&productNumber=239275&brand=SIGALD&PageToGoToURL=https%3A%2F%2Fwww.sigmaald775
rich.com%2Fcatalog%2Fproduct%2Fsigald%2F239275%3Flang%3Den
776
777
[SA] Sigma-Aldrich Inc. 2020b. Phosphoric acid sds [accessed 2021 May 7 2021]. 778
https://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en779
&productNumber=695017&brand=SIGALD&PageToGoToURL=https%3A%2F%2Fwww.sigmaald780
rich.com%2Fcatalog%2Fproduct%2Fsigald%2F695017%3Flang%3Den
781
782
[SA] Sigma-Aldrich Inc. 2020c. Polyphosphoric acid sds [accessed 2021 May 7 2021]. 783
https://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en784
&productNumber=208213&brand=SIGALD&PageToGoToURL=https%3A%2F%2Fwww.sigmaald785
rich.com%2Fcatalog%2Fproduct%2Fsigald%2F208213%3Flang%3Den
786
787
[SA] Sigma-Aldrich Inc. 2021. Pyrophosphoric acid sds [accessed 2021 May 7 2021]. 788
https://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en789
&productNumber=60352&brand=ALDRICH&PageToGoToURL=https%3A%2F%2Fwww.sigmaal790
drich.com%2Fcatalog%2Fproduct%2Faldrich%2F60352%3Flang%3Den
791
792
Silberberg MS. 2003. Chemistry: The Molecular Nature of Matter and Change. 3rd ed. New York (NY): 793
McGraw-Hill Higher Education. 794
795
Timberlake KC. 2016. General, organic, and biological chemistry: structures of life. 5th ed. Hoboken (NJ): 796
Pearson Education Inc. 797

Technical Evaluation Report Phosphoric acid Handling/Processing
August 4, 2021 Page 18 of 18
798
[USDA] United States Department of Agriculture. 2002. Phosphoric acid petition. [accessed 2021 Mar 25]. 799
https://www.ams.usda.gov/sites/default/files/media/Phosphoric%20Acid%202002.pdf
800
801
[USDA] United States Department of Agriculture. 2003. Phosphoric acid technical evaluation report. 802
[accessed 2021 Mar 25]. 803
https://www.ams.usda.gov/sites/default/files/media/Phos%20acid%202002%20technical%20ad804
visory%20panel%20report.pdf
805
806
[USDA] United States Department of Agriculture. 2019. Phosphoric acid petition. [accessed 2021 Mar 25]. 807
https://www.ams.usda.gov/sites/default/files/media/Petition_Phos%20Acid_12_03_2019.pdf
808
809
[USDA] United States Department of Agriculture. 2020a. Phosphoric acid petition. [accessed 2021 Mar 25]. 810
https://www.ams.usda.gov/sites/default/files/media/PetitionAddendum1PhosphoricAcid0407811
2020.pdf
812
813
[USDA] United States Department of Agriculture. 2020b. Phosphoric acid petition. [accessed 2021 Mar 25]. 814
https://www.ams.usda.gov/sites/default/files/media/PetitionAddendum2_PhosphoricAcid_08815
072020.pdf
816
817
Winniczuk PP, Parish ME. 1997. Minimum inhibitory concentrations of antimicrobials against micro-818
organisms related to citrus juice. Food Microbiology. 14: 373-381. 819
820
Wolke RL. 2002. What Einstein told his cook: kitchen science explained. New York (NY): W. W. Norton & 821
Company Inc. 822
823
Yao Y, Xiang H, You L, Cui C, Sun-Waterhouse D, Zhao M. 2017. Hypolipidaemic and antioxidant 824
capacities of polysaccharides obtained from Laminaria japonica by different extraction media in diet-825
induced mouse model. International Journal of Food Science and Technology. 52: 2274-2281. 826
827
Yoon EJ, Lee MY, Choi BI, Lim KJ, Hong SY, Park D. 2020. Pharmaceutical advantages of GenoTX-407, a 828
combination of extracts from Scutellaria baicalensis and Magnolia officinalis bark. Antioxidants. 9: 829
1111. 830
831
Zeng J, Tong Z, Wang L, Zhu JY, Ingram L. 2014. Isolation and structural characterization of sugarcane 832
bagasse lignin after dilute phosphoric acid plus steam explosion pretreatment and its effect on 833
cellulose hydrolysis. Bioresource Technology. 154: 274-281. 834
