EPA Document# EPA-740-R-20-010
August 2020
United States Office of Chemical Safety and
Environmental Protection Agency Pollution Prevention
Final Scope of the Risk Evaluation for
Triphenyl Phosphate
(TPP)
CASRN 115-86-6
August 2020

2
TABLE OF CONTENTS
ACKNOWLEDGEMENTS ......................................................................................................................6
ABBREVIATIONS AND ACRONYMS ..................................................................................................7
EXECUTIVE SUMMARY .......................................................................................................................9
1 INTRODUCTION ............................................................................................................................12
2 SCOPE OF THE EVALUATION ...................................................................................................12
2.1 Reasonably Available Information ..............................................................................................12
Search of Gray Literature ...................................................................................................... 13
Search of Literature from Publicly Available Databases (Peer-Reviewed Literature) .......... 14
Search of TSCA Submissions ................................................................................................ 24
2.2 Conditions of Use ........................................................................................................................24
Categories and Subcategories of Conditions of Use Included in the Scope of the Risk
Evaluation .............................................................................................................................. 25
Activities Excluded from the Scope of the Risk Evaluation ................................................. 27
Production Volume ................................................................................................................ 28
Overview of Conditions of Use and Lifecycle Diagram ....................................................... 28
2.3 Exposures ....................................................................................................................................30
Physical and Chemical Properties ......................................................................................... 30
Environmental Fate and Transport ........................................................................................ 32
Releases to the Environment ................................................................................................. 32
Environmental Exposures ...................................................................................................... 32
Occupational Exposures ........................................................................................................ 33
Consumer Exposures ............................................................................................................. 34
General Population Exposures ............................................................................................... 34
2.4 Hazards (Effects) .........................................................................................................................35
Environmental Hazards ......................................................................................................... 35
Human Health Hazards .......................................................................................................... 35
2.5 Potentially Exposed or Susceptible Subpopulations ...................................................................35
2.6 Conceptual Models ......................................................................................................................36
Conceptual Model for Industrial and Commercial Activities and Uses ................................ 36
Conceptual Model for Consumer Activities and Uses .......................................................... 38
Conceptual Model for Environmental Releases and Wastes: Potential Exposures and
Hazards .................................................................................................................................. 40
2.7 Analysis Plan ...............................................................................................................................42
Physical and Chemical Properties and Environmental Fate .................................................. 42
Exposure ................................................................................................................................ 43
2.7.2.1 Environmental Releases ................................................................................................. 43
2.7.2.2 Environmental Exposures ............................................................................................... 45
2.7.2.3 Occupational Exposures ................................................................................................. 46
2.7.2.4 Consumer Exposures ...................................................................................................... 48
2.7.2.5 General Population ......................................................................................................... 49
Hazards (Effects) ................................................................................................................... 51
2.7.3.1 Environmental Hazards .................................................................................................. 51
2.7.3.2 Human Health Hazards ................................................................................................... 53

3
Summary of Risk Approaches for Characterization .............................................................. 55
2.8 Peer Review .................................................................................................................................56
REFERENCES .........................................................................................................................................57
APPENDICES ..........................................................................................................................................62
ABBREVIATED METHODS FOR SEARCHING AND SCREENING ................... 62
A.1.1 Search Term Genesis and Chemical Verification ...................................................................62
A.1.2 Publicly Available Database Searches ....................................................................................63
A.1.2.1 Query Strings for the Publicly Available Database Searches on TPP .............................63
A.1.2.2 Data Prioritization for Environmental Hazard, Human Health Hazard, Fate and Physical
Chemistry ................................................................................................................67
A.1.2.3 Data Prioritization for Occupational Exposures and Environmental Releases and
General Population, Consumer and Environmental Exposures ..............................68
A.2.1 Inclusion/Exclusion Criteria ...................................................................................................69
A.2.1.1 PECO for Environmental and Human Health Hazards ...................................................69
A.2.1.2 PECO for Consumer, Environmental, and General Population Exposures .....................71
A.2.1.3 RESO for Occupational Exposure and Environmental Releases ....................................72
A.2.1.4 PESO for Fate and Transport ..........................................................................................74
A.2.1.5 Generation of Hazard Heat Maps ....................................................................................76
A.3.1 Screening of Gray Literature ..................................................................................................77
A.3.2 Initial Screening of Sources using Decision Logic Tree ........................................................78
A.3.3 TSCA Submission Searching and Title Screening .................................................................79
A.3.4 Gray Literature Search Results for TPP .................................................................................80
PHYSICAL AND CHEMICAL PROPERTIES .......................................................... 83
ENVIRONMENTAL FATE AND TRANSPORT PROPERTIES ............................ 84
REGULATORY HISTORY .......................................................................................... 86
PROCESS, RELEASE AND OCCUPATIONAL EXPOSURE INFORMATION .. 91
E.1.1 Manufacturing (Including Import) ..........................................................................................91
E.1.1.1 Domestic Manufacture ....................................................................................................91
E.1.1.2 Import ..............................................................................................................................91
E.1.2 Processing and Distribution ....................................................................................................91
E.1.2.1 Incorporation into a Formulation, Mixture or Reaction Product .....................................91
E.1.2.2 Incorporation into an Article ...........................................................................................91
E.1.2.3 Recycling .........................................................................................................................91
E.1.3 Uses .........................................................................................................................................92
E.1.3.1 Paints and Coatings .........................................................................................................92
E.1.3.2 Plastic and Rubber Products ............................................................................................92

4
E.1.3.3 Laboratory Chemicals .....................................................................................................92
E.1.3.4 Operational Fluids, Maintenance Fluids and Semisolids, Reactive Fluids, and Solids
Used in Aerospace Industry ....................................................................................92
E.1.3.5 Turbine Engine Oils Used in Aviation ............................................................................93
E.1.3.6 Turbine Engine Oils Used in Non-Aviation Industries ...................................................93
E.1.3.7 Foam Seating and Bedding Products ...............................................................................93
E.1.3.8 Furniture and Furnishings ................................................................................................93
E.1.3.9 Lubricants and Greases ....................................................................................................93
E.1.3.10 Electrical and Electronic Products ...................................................................................93
E.1.4 Disposal .....................................................................................................................................93
SUPPORTING INFORMATION – CONCEPTUAL MODEL FOR INDUSTRIAL
AND COMMERCIAL ACTIVITIES AND USES ...................................................... 95
SUPPORTING INFORMATION- CONCEPTUAL MODEL FOR CONSUMER
ACTIVITIES AND USES ............................................................................................ 108
SUPPORTING INFORMATION – CONCEPTUAL MODEL FOR
ENVIRONMENTAL RELEASES AND WASTES ................................................... 112
LIST OF TABLES
Table 2-1. Results of Title Screening of Submissions to EPA under Various Sections of TSCA ........... 24
Table 2-2. Categories and Subcategories of Conditions of Use Included in the Scope of the Risk
Evaluation ......................................................................................................................... 25
Table 2-3. Physical and Chemical Properties of TPP ............................................................................... 30
Table 2-4. Categories and Sources of Environmental Release Data ........................................................ 43
LIST OF FIGURES
Figure 2-1. Gray Literature Search Results for TPP ................................................................................. 14
Figure 2-2. Peer-reviewed Literature Inventory Tree – Physical and Chemical Properties Search Results
for TPP .............................................................................................................................. 15
Figure 2-3. Peer-reviewed Literature Inventory Tree - Fate and Transport Search Results for TPP ....... 16
Figure 2-4. Peer-reviewed Literature Inventory Heat Map - Fate and Transport Search Results for TPP17
Figure 2-5. Peer-reviewed Literature Inventory Tree - Engineering Search Results for TPP .................. 18
Figure 2-6. Peer-reviewed Literature Inventory Heat Map - Engineering Search Results for TPP ......... 19
Figure 2-7. Peer-reviewed and Gray Literature Inventory Tree - Exposure Search Results for TPP ....... 20
Figure 2-8. Peer-reviewed and Gray Literature Inventory Heat Map – Exposure Search Results for TPP
........................................................................................................................................... 21
Figure 2-9. Peer-reviewed Literature Inventory Tree - Human Health and Environmental Hazard Search
Results for TPP ................................................................................................................. 22
Figure 2-10. Peer-reviewed Literature Inventory Heat Map – Human Health and Environmental Hazards
Search Results for TPP ..................................................................................................... 23
Figure 2-11. TPP Life Cycle Diagram ...................................................................................................... 29
Figure 2-12. Box and Whisker Plots of Reported Physical and Chemical Property Values .................... 32
Figure 2-13. TPP Conceptual Model for Industrial and Commercial Activities and Uses: Worker and
ONU Exposures and Hazards ........................................................................................... 37

5
Figure 2-14. TPP Conceptual Model for Consumer Activities and Uses: Consumer Exposures and
Hazards ............................................................................................................................. 39
Figure 2-15. TPP Conceptual Model for Environmental Releases and Wastes: Environmental and
General Population Exposure and Hazards ....................................................................... 41
LIST OF APPENDIX TABLES
Table_Apx A-1. Sources of Verification for Chemical Names and Structures ....................................... 62
Table_Apx A-2. Summary of Data Sources, Search Dates and Number of Peer-Reviewed Literature
Search Results for TPP .................................................................................................. 64
Table_Apx A-3. Hazards Title and Abstract and Full-Text PECO Criteria for TPP ............................... 69
Table_Apx A-4. Major Categories of Potentially Relevant Supplemental Material for TPP .................. 70
Table_Apx A-5. Generic Inclusion Criteria for the Data Sources Reporting Exposure Data on General
Population, Consumers and Environmental Receptors ................................................. 71
Table_Apx A-6. Pathways Identified as Supplemental for TPP
a
............................................................. 72
Table_Apx A-7. Inclusion Criteria for Data Sources Reporting Engineering and Occupational Exposure
Data ............................................................................................................................... 72
Table_Apx A-8. Engineering, Environmental Release and Occupational Data Necessary to Develop the
Environmental Release and Occupational Exposure Assessments ............................... 73
Table_Apx A-9. Inclusion Criteria for Data or Information Sources Reporting Environmental Fate and
Transport Data ............................................................................................................... 75
Table_Apx A-10. Fate Endpoints and Associated Processes, Media and Exposure Pathways Considered
in the Development of the Environmental Fate Assessment ....................................... 75
Table_Apx A-11. Decision Logic Tree Overview .................................................................................... 78
Table_Apx A-12. Gray Literature Sources that Yielded Results for TPP ................................................ 80
Table_Apx B-1. Summary Statistics for Reviewed Physical Properties .................................................. 83
Table_Apx C-1 Environmental Fate and Transport Properties of TPP .................................................... 84
Table_Apx D-1 Federal Laws and Regulations ........................................................................................ 86
Table_Apx D-2. State Laws and Regulations ........................................................................................... 87
Table_Apx D-3 Regulatory Actions by other Governments, Tribes, and International Agreements ....... 89
Table_Apx E-1. Potentially Relevant Data Sources for Exposure Monitoring and Area Monitoring Data
from NIOSH Health Hazard Evaluations for TPP
a
........................................................ 94
Table_Apx F-1. Worker and Occupational Non-User Exposure Conceptual Model Supporting Table .. 95
Table_Apx G-1. Consumer Exposure Conceptual Model Supporting Table ......................................... 108
Table_Apx H-1. General Population and Environmental Exposure Conceptual Model Supporting Table
......................................................................................................................................... 112
LIST OF APPENDIX FIGURES
Figure_Apx A-1. Decision Logic Tree Used to Screen Gray Literature Results ..................................... 78

6
ACKNOWLEDGEMENTS
This report was developed by the United States Environmental Protection Agency (U.S. EPA), Office of
Chemical Safety and Pollution Prevention (OCSPP), Office of Pollution Prevention and Toxics (OPPT).
Acknowledgements
The OPPT Assessment Team gratefully acknowledges participation or input from intra-agency
reviewers that included multiple offices within EPA, inter-agency reviewers that included multiple
federal agencies, and assistance from EPA contractors GDIT (Contract No. HHSN316201200013W),
ERG (Contract No. EP-W-12-006), Versar (Contract No. EP-W-17-006), ICF (Contract
No.68HERC19D0003), Abt Associates (Contract No. EP-W-16-009) and SRC (Contract No.
68HERH19F0213). EPA also acknowledges the contributions of technical experts from EPA’s Office of
Research and Development.
Docket
Supporting information can be found in public docket: EPA-HQ-OPPT-2018-0458.
Disclaimer
Reference herein to any specific commercial products, process or service by trade name, trademark,
manufacturer or otherwise does not constitute or imply its endorsement, recommendation or favoring by
the United States Government.

7
ABBREVIATIONS AND ACRONYMS
ADME Absorption, Distribution, Metabolism, and Excretion
ATSDR Agency for Toxic Substances and Disease Registry
BAF Bioaccumulation Factor
BCF Bioconcentration Factor
BMF Biomagnification factor
BOD Biochemical oxygen demand
CAA Clean Air Act
CASRN Chemical Abstracts Service Registry Number
CBI Confidential Business Information
CCL Contaminant Candidate List
CDR Chemical Data Reporting
CFR Code of Federal Regulations
ChemSTEER Chemical Screening Tool for Exposure and Environmental Releases
CSF Cancer Slope Factor
CWA Clean Water Act
EC Engineering control
ECHA European Chemicals Agency
EPA Environmental Protection Agency
ESD Emission Scenario Document
FYI For Your Information
GS Generic Scenario
HAP Hazardous Air Pollutant
HSDB Hazardous Substances Data Bank
ILO International Labour Organization
IUR
Inventory Update Rule
IURs Inhalation Unit Risks
K
Thousand
K
OC
Organic Carbon: Water Partition Coefficient
K
OW
Octanol: Water Partition Coefficient
M Million
MOE Margins of Exposure
MITI Ministry of International Trade and Industry
NICNAS National Industrial Chemicals Notification and Assessment Scheme
NIH National Institutes of Health
NIOSH National Institute for Occupational Safety and Health
OECD Organisation for Economic Co-operation and Development
OH Hydroxyl radical
OSF Oral Slope Factor
OSHA Occupational Safety and Health Administration
P Persistence
PBPK Physiologically Based Pharmacokinetic
PBT Persistent, Bioaccumulative, Toxic
PECO Population, Exposure, Comparator and Outcome
PEL Permissible Exposure Limit
PESO Pathways and Processes, Exposure, Setting or Scenario, and Outcomes
PESS Potentially Exposed or Susceptible Subpopulation

8
PNOR Particulates Not Otherwise Regulated
POD Point Of Departure
PPE Personal Protective Equipment
RCRA Resource Conservation and Recovery Act
RESO Receptors, Exposure, Setting or Scenario, and Outcomes
SDWA Safe Drinking Water Act
SIDS Screening Information Data Sets
SMILES Simplified molecular-input line-entry system
SVOC Semi-volatile organic compound
STEL Short-term Exposure Limit
TIAB Title and abstract
TLV Threshold Limit Value
TMF Trophic Magnification Factors
TRI Toxics Release Inventory
TSCA Toxic Substances Control Act
TWA Time-weighted average
UCMR Unregulated Contaminants Monitoring Rule
VP Vapor Pressure
WS Water solubility

9
EXECUTIVE SUMMARY
In December 2019, EPA designated Triphenyl Phosphate (TPP) (CASRN 115-86-6) as a high-priority
substance for risk evaluation following the prioritization process required by Section 6(b) of the Toxic
Substances Control Act (TSCA) and implementing regulations (40 CFR Part 702) (Docket ID: EPA-
HQ-OPPT-2019-0131). The first step of the risk evaluation process is the development of the draft scope
document. EPA published the Draft Scope of the Risk Evaluation for Triphenyl Phosphate (CASRN 115-
86-6) (EPA Document No. EPA-740-D-20-010) (U.S. EPA, 2020c) and provided a 45-day comment
period on the draft scope per 40 CFR 702.41(c)(7). EPA has considered comments received (Docket ID:
EPA-HQ-OPPT-2018-0458) during the public comment period to inform the development of this final
scope document, and public comments received will continue to inform the development of the risk
evaluation for TPP. This document fulfills the TSCA requirement to issue a final scope document per
TSCA Section 6(b)(4)(D) and as described in 40 CFR 702.41(c)(8). The scope for TPP includes the
following information: the conditions of use, potentially exposed or susceptible subpopulations (PESS),
hazards, and exposures that EPA plans to consider in the risk evaluation, along with a description of the
reasonably available information, conceptual model, analysis plan and science approaches, and plan for
peer review for this chemical substance.
General Information. TPP is a colorless solid that is primarily used as a flame retardant with a total
production volume in the United States between 1 million and 10 million pounds.
Reasonably Available Information. EPA leveraged the data and information sources already described
in the Proposed Designation of Triphenyl Phosphate (CASRN 115-86-6) as a High-Priority Substance
for Risk Evaluation (U.S. EPA, 2019d) to inform the development of this scope document. Furthermore,
EPA conducted a comprehensive search to identify and screen multiple evidence streams (i.e.,
chemistry, fate, release and engineering, exposure, hazard), and the search and screening results to date
are provided in Section 2.1. EPA used the systematic review process described in Appendix A to search
for and screen reasonably available information, including information already in EPA’s possession, for
inclusion in the risk evaluation. This information includes the hazards, exposures, PESS, and conditions
of use that may help inform the risk evaluation for TPP. EPA has focused on the data collection phase
(consisting of data search, data screening, and data extraction) during the preparation of the scope
document, whereas the data evaluation and integration stages will occur during the development of the
risk evaluation and thus are not part of the scoping activities described in this document. EPA will
consider additional information identified following publication of this scope document, as appropriate,
in developing the risk evaluation, including the Chemical Data Reporting (CDR) information that the
Agency will receive by the end of November 2020.
Conditions of Use. EPA plans to evaluate manufacturing (including importing); processing; distribution
in commerce; industrial, commercial and consumer uses; and disposal of TPP in the risk evaluation. TPP
is manufactured (including imported) in the United States. The chemical is processed as a reactant;
incorporated into formulation, mixture, or reaction products; and incorporated into articles. Several
commercial uses were identified, such as paints and coatings and plastic and rubber products. Consumer
uses were reported in foam seating and bedding products. EPA identified these conditions of use from
information reported to EPA through CDR, published literature, and consultation with stakeholders for
both uses currently in production and uses whose production may have ceased. EPA revised the
conditions of use in the final scope of the risk evaluation based on additional information and public
comments (Docket ID: EPA-HQ-OPPT-2018-0458) on the draft scope document. EPA is aware of
10
information reporting use of TPP in nail polish and in flea and tick collars; however, these are not
conditions of use for the chemical substance as defined in TSCA § 3(2) and (4).
Conceptual Model. The conceptual models for TPP are presented in Section 2.6. Conceptual models are
graphical depictions of the actual or predicted relationships of conditions of use, exposure pathways
(e.g., media), exposure routes (e.g., inhalation, dermal, oral), hazards and receptors throughout the life
cycle of the chemical substance. EPA considered reasonably available information as well as public
comments received on the draft scope document for TPP in finalizing the exposure pathways, exposure
routes, and hazards EPA plans to evaluate in the risk evaluation. As a result, EPA plans to focus the risk
evaluation for TPP on the following exposures, hazards, and receptors:
• Exposures (Pathways and Routes), Receptors and PESS. EPA plans to evaluate releases to the
environment as well as human and environmental exposures resulting from the conditions of use
of TPP that EPA plans to consider in risk evaluation. Exposures to TPP are discussed in Section
2.3. Additional information gathered through systematic review searches will also inform
expected exposures.
EPA’s plan for evaluating environmental exposure pathways in the scope of the risk evaluation
considers whether other EPA administered statutes and regulatory programs cover TPP in media
pathways falling under the jurisdiction of those authorities. TPP does not have pathways covered
under the jurisdiction of other EPA-administered laws. In Section 2.6, EPA presents the
conceptual models describing the identified exposures (pathways and routes), receptors and
hazards associated with the conditions of use of TPP within the scope of the risk evaluation.
EPA considered reasonably available information and comments received on the draft scope for
TPP in determining the human and environmental exposure pathways, routes, receptors and
PESS for inclusion in the final scope. EPA plans to evaluate the following human and
environmental exposure pathways, routes, receptors and PESS in the scope of the risk
evaluation:
‒ Occupational exposure: EPA plans to evaluate exposures to workers and occupational
non-users (ONUs) via the inhalation route and exposures to workers via the dermal route
associated with manufacturing, import, processing, use and disposal of TPP.
‒ Consumer and bystander exposure: EPA plans to evaluate oral and dermal exposure to
TPP for consumers, and inhalation exposure to bystanders and consumers from use of
foam and upholstery, automobile upholstery, camping tents, thermoplastic products,
vulcanization products, hydraulic fluids containing TPP; and children’s mouthing of
products/articles containing TPP.
‒ General population exposure:EPA plans to evaluate general population exposure to TPP
via the oral route from drinking water, surface water, groundwater, fish ingestion, human
breast milk and soil, via the inhalation route from ambient air and via dermal route from
contact with drinking water, surface water, groundwater and soil.
‒ PESS: EPA plans to evaluate children, women of reproductive age (e.g., pregnant
women, breast-feeding women), workers and consumers as PESS in the risk evaluation.
‒ Environmental exposure: EPA plans to evaluate exposure to TPP for aquatic and
terrestrial receptors.

11
• Hazards. Hazards for TPP are discussed in Section 2.4. EPA completed preliminary reviews of
information (e.g., federal and international government chemical assessments) to identify
potential environmental and human health hazards for TPP as part of the prioritization (U.S.
EPA, 2019d) and scoping process (U.S. EPA, 2020c). EPA also considered reasonably available
information collected through systematic review methods as outlined in Appendix A and public
comments received on the draft scope for TPP in determining the broad categories of
environmental and human health hazard effects to be evaluated in the risk evaluation. EPA will
use systematic review methods to evaluate the epidemiological and toxicological literature for
TPP.
EPA plans to evaluate all potential environmental and human health hazard effects identified for
TPP in Sections 2.4.1 and 2.4.2, respectively. Identified through the data screening phase of
systematic review, the potential environmental hazard effects and related information that EPA
plans to consider for the risk evaluation include: ADME, PBPK, cancer, cardiovascular,
developmental, endocrine, gastrointestinal, hematological and immune, hepatic, mortality,
musculoskeletal, neurological, nutritional and metabolic, ocular and sensory, reproductive,
respiratory and skin and connective tissue for TPP. Similarly, the potential human health hazard
effects and related information identified through prioritization and the data screening phase of
systematic review for TPP that EPA plans to consider for the risk evaluation include: ADME,
cancer, cardiovascular, developmental, endocrine, gastrointestinal, hematological and immune,
hepatic, mortality, musculoskeletal, neurological, nutritional and metabolic, ocular and sensory,
renal, reproductive and skin and connective tissue.
Analysis Plan. The analysis plan for TPP is presented in Section 2.7. The analysis plan outlines the
general science approaches that EPA plans to use for the various evidence streams (i.e., chemistry, fate,
release and engineering, exposure, hazard) supporting the risk evaluation. The analysis plan is based on
EPA’s knowledge of TPP to date which includes review of identified information as described in
Section 2.1. Should additional data or approaches become reasonably available, EPA may consider them
for the risk evaluation.
Peer Review. The draft risk evaluation for TPP will be peer reviewed. Peer review will be conducted in
accordance with relevant and applicable methods for chemical risk evaluations, including using EPA’s
Peer Review Handbook (U.S. EPA, 2015b) and other methods consistent with Section 26 of TSCA (see
40 CFR 702.45).
12
1 INTRODUCTION
This document presents the scope of the risk evaluation to be conducted for TPP under the Frank R.
Lautenberg Chemical Safety for the 21st Century Act. The Frank R. Lautenberg Chemical Safety for the
21st Century Act amended TSCA on June 22, 2016. The new law includes statutory requirements and
deadlines for actions related to conducting risk evaluations of existing chemicals.
Under TSCA § 6(b), the Environmental Protection Agency (EPA) must designate chemical substances
as high-priority substances for risk evaluation or low-priority substances for which risk evaluations are
not warranted at the time, and upon designating a chemical substance as a high-priority substance,
initiate a risk evaluation on the substance. TSCA § 6(b)(4) directs EPA to conduct risk evaluations for
existing chemicals to "determine whether a chemical substance presents an unreasonable risk of injury
to health or the environment, without consideration of costs or other non- risk factors, including an
unreasonable risk to a potentially exposed or susceptible subpopulation identified as relevant to the risk
evaluation by the Administrator, under the conditions of use."
TSCA § 6(b)(4)(D) and implementing regulations require that EPA publish the scope of the risk
evaluation to be conducted, including the hazards, exposures, conditions of use and PESS that the
Administrator expects to consider, within 6 months after the initiation of a risk evaluation. In addition, a
draft scope is to be published pursuant to 40 CFR 702.41. In December 2019, EPA published a list of 20
chemical substances that have been designated high priority substances for risk evaluations (Docket ID:
EPA-HQ-OPPT-2019-0131) (84 FR 71924, December 30, 2019), as required by TSCA § 6(b)(2)(B),
which initiated the risk evaluation process for those chemical substances. TPP is one of the chemicals
designated as a high priority substance for risk evaluation. On April 9, 2020, EPA published the Draft
Scope of the Risk Evaluation for TPP (EPA Document No. 740-D-20-010) (85 FR 19941, April 9, 2020)
(U.S. EPA, 2020c) for a 45-day public comment period. After reviewing and considering the public
comments received (Docket ID: EPA-HQ-OPPT-2018-0458) on the draft scope document, EPA is now
publishing this final scope document pursuant to 40 CFR 702.41(c)(8).
2 SCOPE OF THE EVALUATION
2.1 Reasonably Available Information
EPA conducted a comprehensive search for reasonably available information
1
to support the
development of this final scope document for TPP. EPA leveraged the data and information sources
already collected in the documents supporting the chemical substance’s high-priority substance
designation. In addition, EPA searched for additional data and information on physical and chemical
properties, environmental fate, engineering, exposure, environmental and human health hazards that
could be obtained from the following general categories of sources:
1. Databases containing publicly available, peer-reviewed literature;
2. Gray literature, which is defined as the broad category of data/information sources not found in
standard, peer-reviewed literature databases;
1
Reasonably available information means information that EPA possesses or can reasonably generate, obtain, and synthesize
for use in risk evaluations, considering the deadlines specified in TSCA Section 6(b)(4)(G) for completing such evaluation.
Information that meets the terms of the preceding sentence is reasonably available information whether or not the information
is confidential business information, that is protected from public disclosure under TSCA Section 14. (40 CFR 702.33).

13
3. Data and information submitted under TSCA Sections 4, 5, 8(e), and 8(d), as well as “for your
information” (FYI) submissions.
Following the comprehensive search, EPA performed a title and abstract screening to identify
information potentially relevant for the risk evaluation process. This step also classified the references
into useful categories or tags to facilitate the sorting of information through the systematic review
process.
Search terms were used to search each of the literature streams and gather TPP studies. These terms and
the methods used to develop them are listed in Appendix A. The studies resulting from the search
process were loaded into the EPA Health and Environmental Research Online (HERO) database and
then prioritized to screen first the literature likely relevant for each of the disciplines: fate, physical/
chemical properties, engineering, exposure and hazard. The tools and methods used to manage the
screening process are also outlined in Appendix A. The studies resulting from the search underwent a
title/abstract screening process, which tagged them by topic or category. Following this, a determination
was made to move studies forward into full-text screening. The criteria used in the screening process for
each discipline are found in the population, exposure comparator, outcome (PECO) statements listed in
Appendix A. The screening process results are presented in the form of literature inventory trees and
heat maps in Section 2.1.2. The screening process was conducted based on EPA’s planning, execution
and assessment activities outlined in Appendix A.
EPA has focused on the data collection phase (consisting of data search, data screening, and data
extraction) during the preparation of the scope document, whereas the data evaluation and integration
stages will occur during the development of the risk evaluation and thus are not part of the scoping
activities described in this document.
The subsequent sections summarize the data collection activities completed up to date for the general
categories of sources and topic areas (or disciplines) using systematic review methods.
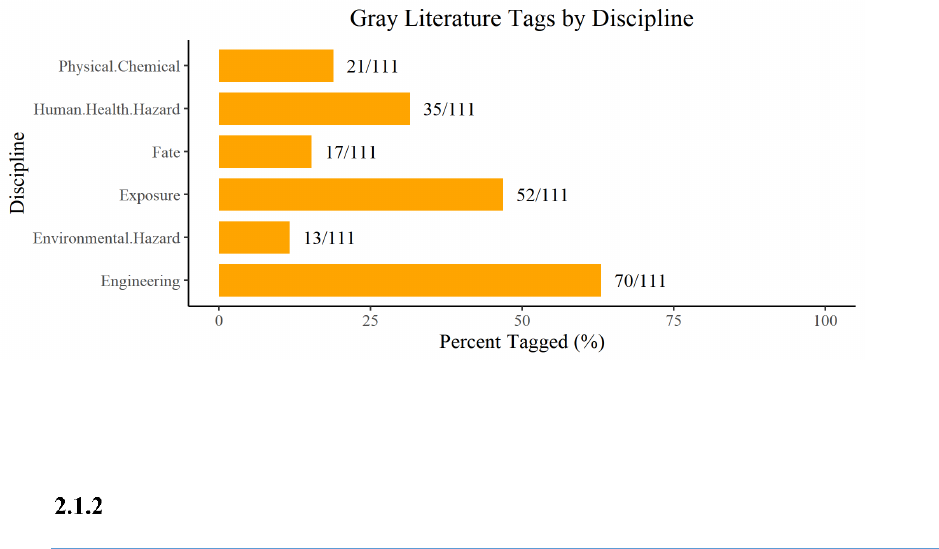
Search of Gray Literature
EPA surveyed the gray literature
2
and identified 111 search results relevant to EPA's risk evaluation
needs for TPP. Appendix A.3.4 lists the gray literature sources that yielded 111 discrete data or
information sources relevant to TPP. EPA further categorized the data and information into the various
topic areas (or disciplines) supporting the risk evaluation (e.g., physical and chemical properties,
environmental fate, environmental hazard, human health hazard, exposure, engineering), and the
breakdown is shown in Figure 2-1. EPA will consider additional reasonably available information from
gray literature if it becomes available during the risk evaluation phase.
2
Gray literature is defined as the broad category of data/information sources not found in standard, peer-reviewed literature
databases (e.g., PubMed and Web of Science). Gray literature includes data/information sources such as white papers,
conference proceedings, technical reports, reference books, dissertations, information on various stakeholder websites, and
other databases.
14
Figure 2-1. Gray Literature Search Results for TPP
The percentages across disciplines do not add up to 100%, as each source may provide data or
information for various topic areas (or disciplines).
Search of Literature from Publicly Available Databases (Peer-Reviewed
Literature)
EPA has begun the systematic review process and has conducted searching and screening of the
reasonably available literature using the process outlined in Appendix A. This includes performing a
comprehensive search of the reasonably available peer review literature on physical and chemical
properties, environmental fate and transport, engineering (environmental release and occupational
exposure), exposure (environmental, general population and consumer) and environmental and human
health hazards of TPP. Eligibility criteria were applied in the form of PECO statements (see Appendix
A). Included references met the PECO or similar criteria, whereas excluded references did not meet the
criteria (i.e., not relevant), and supplemental material was considered as potentially relevant (see
Appendix A.2.). EPA plans to evaluate the reasonably available information identified for each
discipline during the development of the risk evaluation.
EPA created literature inventory trees to graphically illustrate the flow of data and information sources
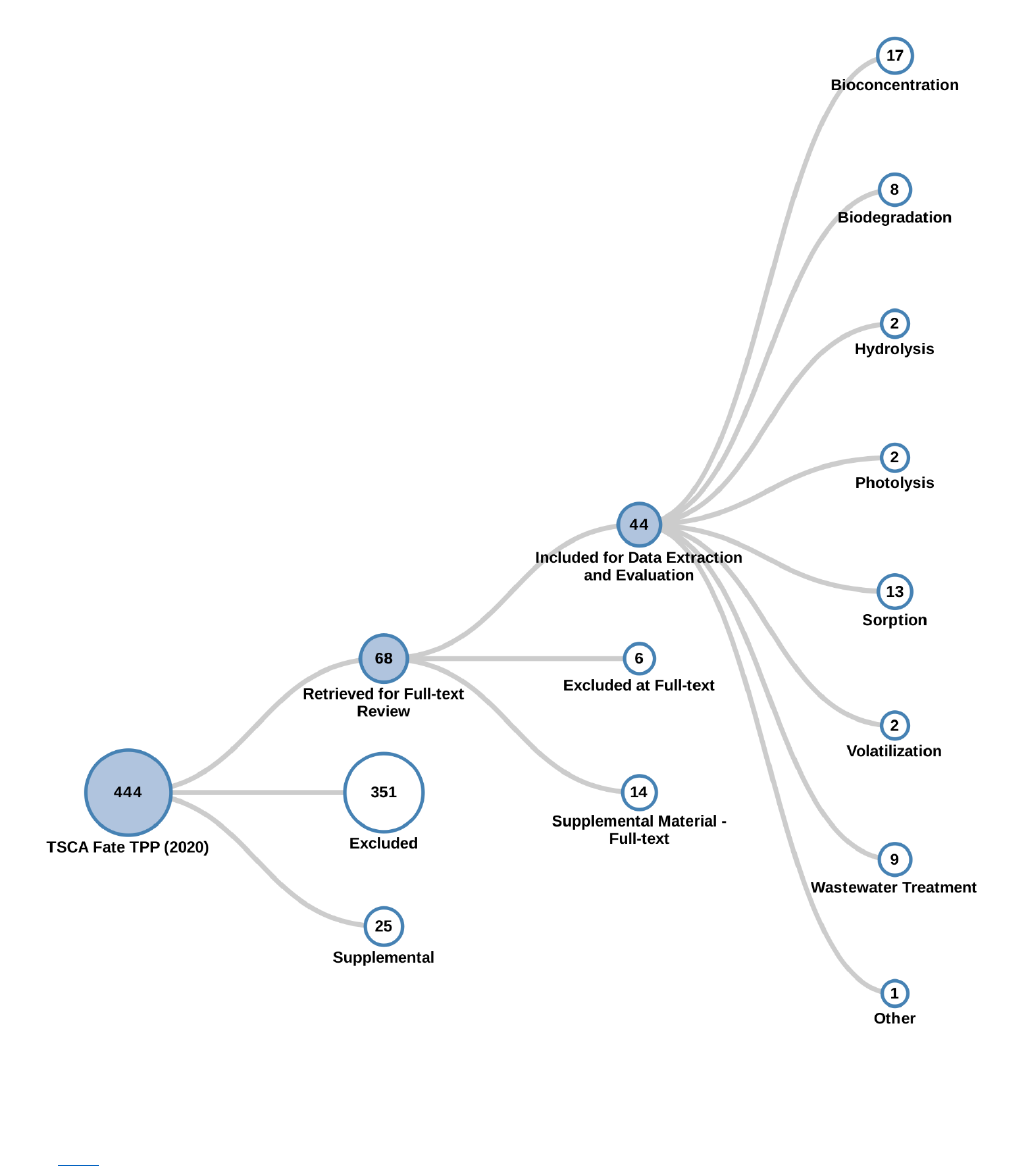
following full-text screening (see Figure 2-2, Figure 2-3, Figure 2-5, Figure 2-7, and Figure 2-9). For the
physical and chemical, fate, engineering and hazard literature, EPA used the Health Assessment
Workplace Collaborative (HAWC) tool to develop web-based literature inventory trees illustrating,
through interactive links, studies that were included or excluded. These literature inventory trees
enhance the transparency of the decisions resulting from the screening process described in Appendix A.
For each of the corresponding disciplines, the literature was tagged for evaluation during the risk
evaluation. Literature inventory trees for physical and chemical properties and for exposure are provided
as static diagrams (Figure 2-2). For all other disciplines, static screen captures are provided in addition
to links to the interactive trees, which are provided in their corresponding captions. The links show
individual studies that were tagged as included, excluded, or supplemental. Supplemental studies did not
meet all inclusion criteria but may be considered during risk evaluation as supporting information
(Appendix A). These studies can be accessed through the hyperlink provided in the associated caption.
In some figures, the sum of the numbers for the various sub-categories may be larger than the broader
category because some studies may be included under multiple sub-categories. In other cases, the sum of
15
the various sub-categories may be smaller than the main category because some studies may not be
depicted in the sub-categories if their relevance to the risk evaluation was unclear.
In addition, EPA tabulated the number and characteristics of the data and information sources included
in the full-text screening process in the form of a literature inventory heat map for the fate, engineering,
exposure and hazard information (see Figure 2-4, Figure 2-6, Figure 2-8 and
Figure 2-10). For each of these four disciplines, a static image of the literature inventory heat map is
provided, and a link to the interactive version presented in HAWC is included in the caption below each
diagram.
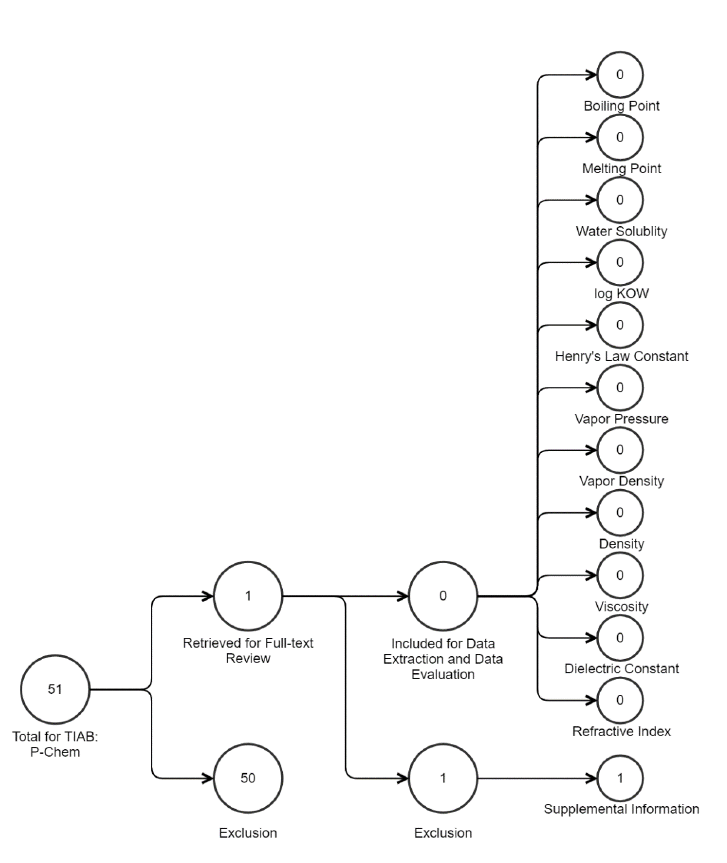
Figure 2-2. Peer-reviewed Literature Inventory Tree – Physical and Chemical Properties Search
Results for TPP
Data in this static figure represent references obtained from the publicly available databases search (see
Appendix A.1.2) that were included during full-text screening as of June 2, 2020. TIAB refers to “title
and abstract” screening
16
Figure 2-3. Peer-reviewed Literature Inventory Tree – Fate and Transport Search Results for
TPP
Click here to view the interactive literature inventory tree. Data in this figure represent references
obtained from the publicly available databases search (see Appendix A.1.2) that were included during
full-text screening as of June 2, 2020. Additional data may be added to the interactive version as they
become available.
17
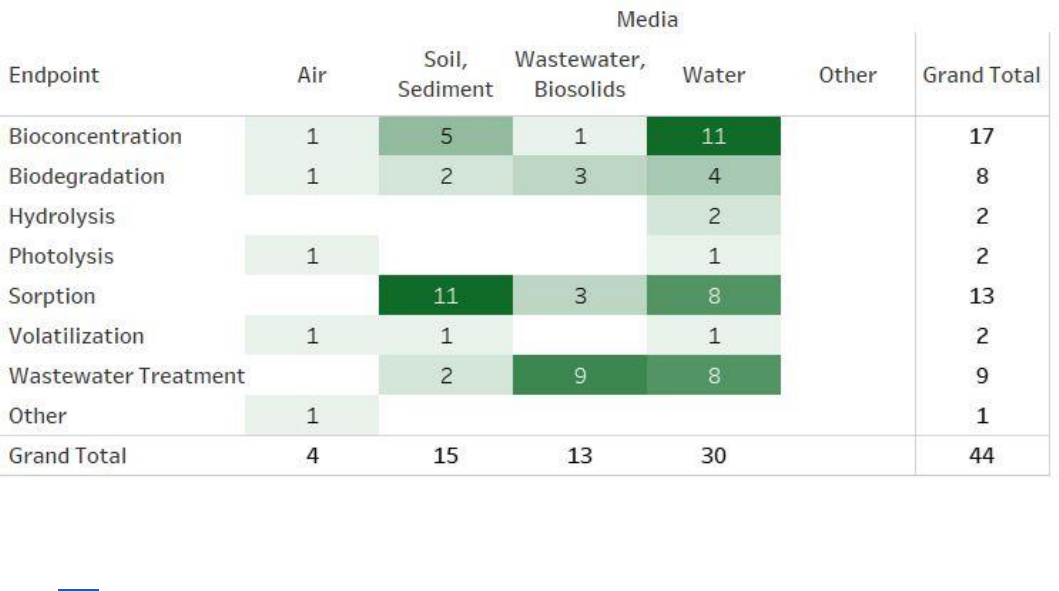
Figure 2-4. Peer-reviewed Literature Inventory Heat Map – Fate and Transport Search Results
for TPP
Click here to view the interactive version for additional study details. The column totals, row totals, and
grand totals indicate total numbers of unique references, as some references may be included in multiple
cells. The various shades of color green visually represent the number of relevant references identified
by media or endpoint. The darker the color, the more references are available for a given media or
endpoint. Data in this figure represents references obtained from the publicly available databases search
(see Appendix A.1.2) that were included during full-text screening as of June 2, 2020. Additional data
may be added to the interactive version as they become available.
18
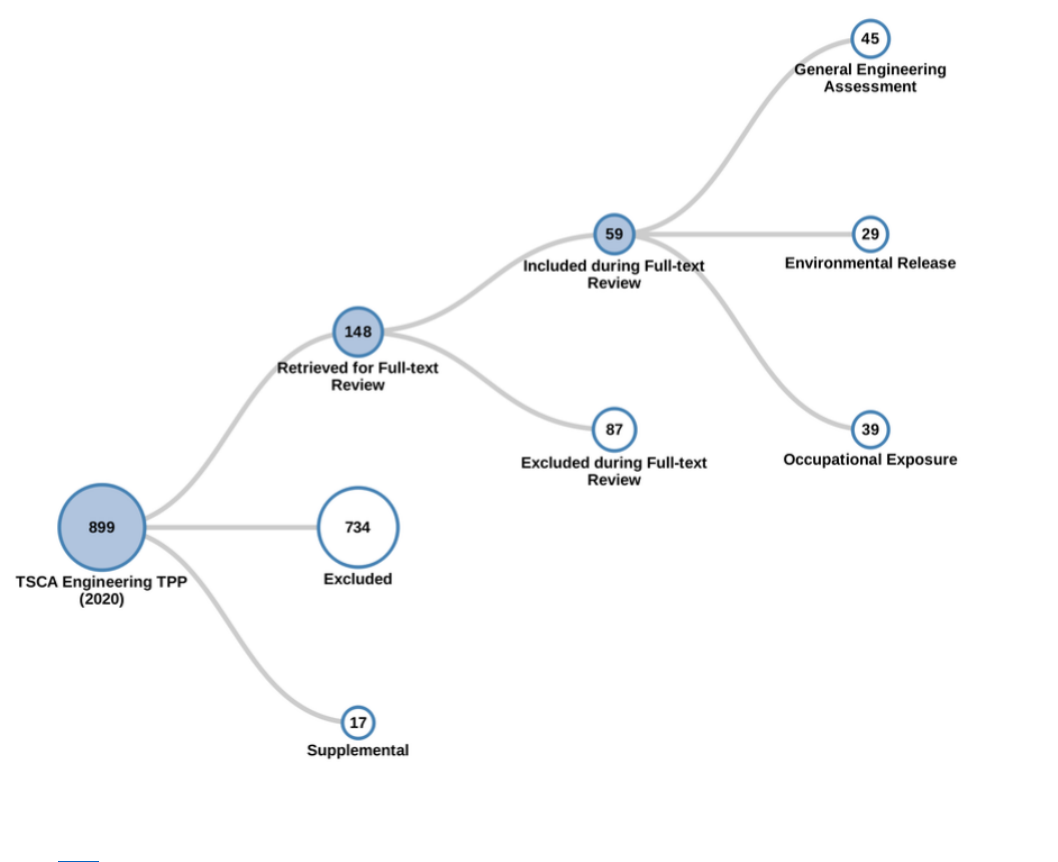
Figure 2-5. Peer-reviewed Literature Inventory Tree – Engineering Search Results for TPP
Click here to view the interactive literature inventory tree. Data in this figure represent references
obtained from the publicly available databases search (see Appendix A.1.2.) that were included during
full-text screening as of August 5, 2020. Additional data may be added to the interactive version as they
become available.
19
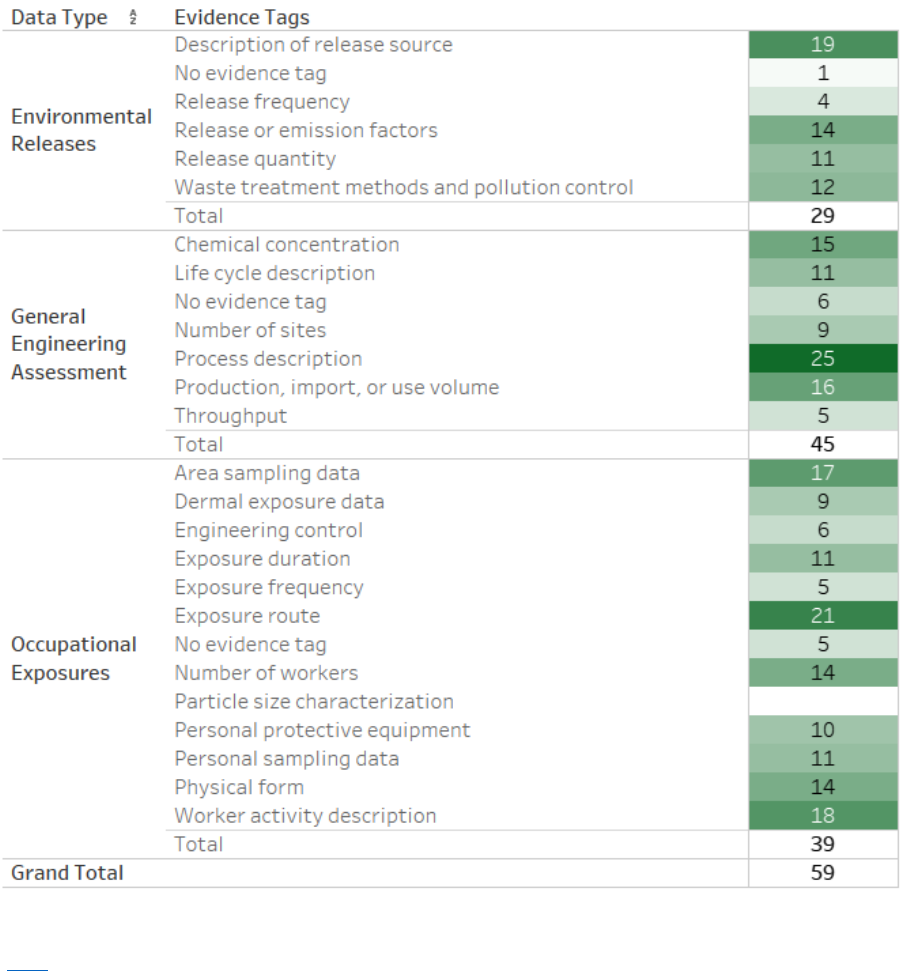
Figure 2-6. Peer-reviewed Literature Inventory Heat Map – Engineering Search Results for TPP
Click here to view the interactive version for additional study details. Data in this figure represent
references obtained from the publicly available databases search (see Appendix A.1.2) that were
included during full-text screening as of August 5, 2020. Additional data may be added to the interactive
version as they become available.
20
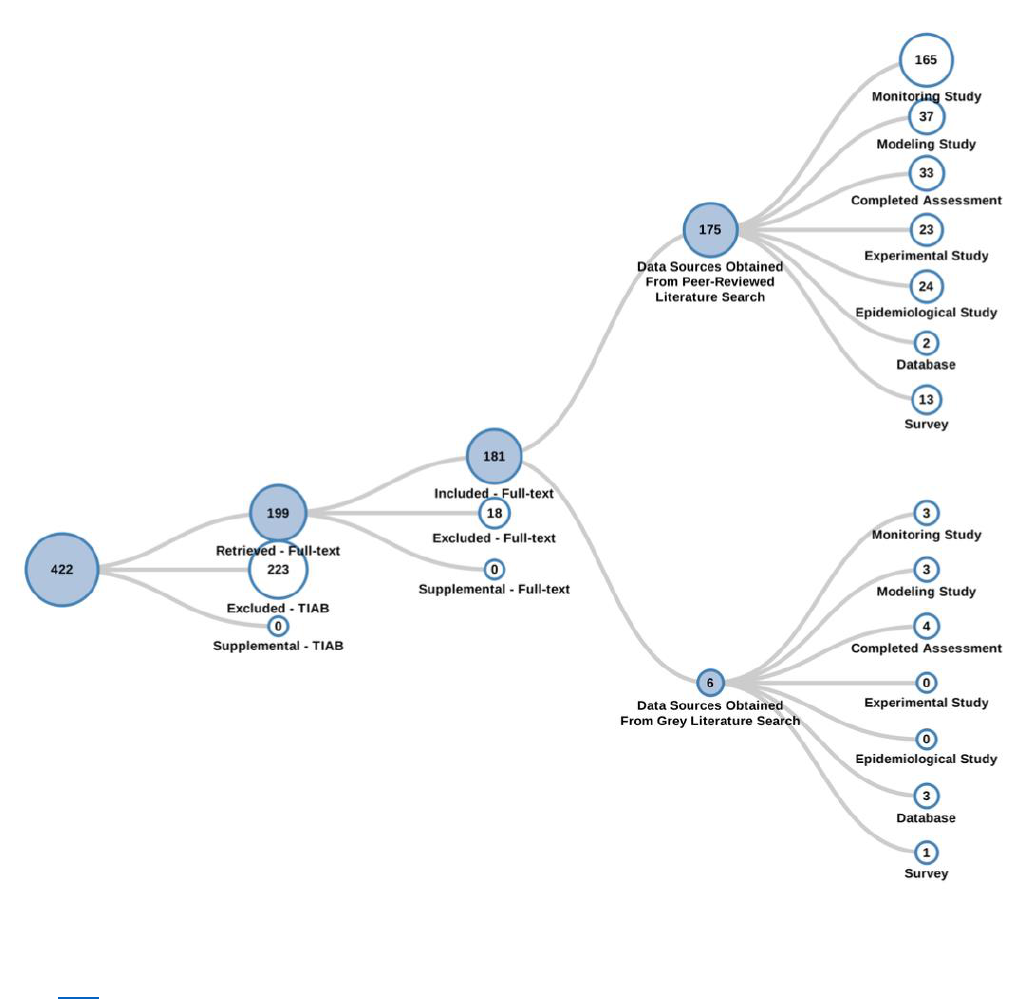
Figure 2-7. Peer-reviewed and Gray Literature Inventory Tree – Exposure Search Results for
TPP
Click here to view the interactive literature inventory tree. Data in this figure represent all references
obtained from the publicly available databases search (see Appendix A.1.2), and gray literature
references search (see Appendix A.3) that were included during full-text screening as of July 31, 2020.
Additional data may be added to the interactive version as they become available.
21
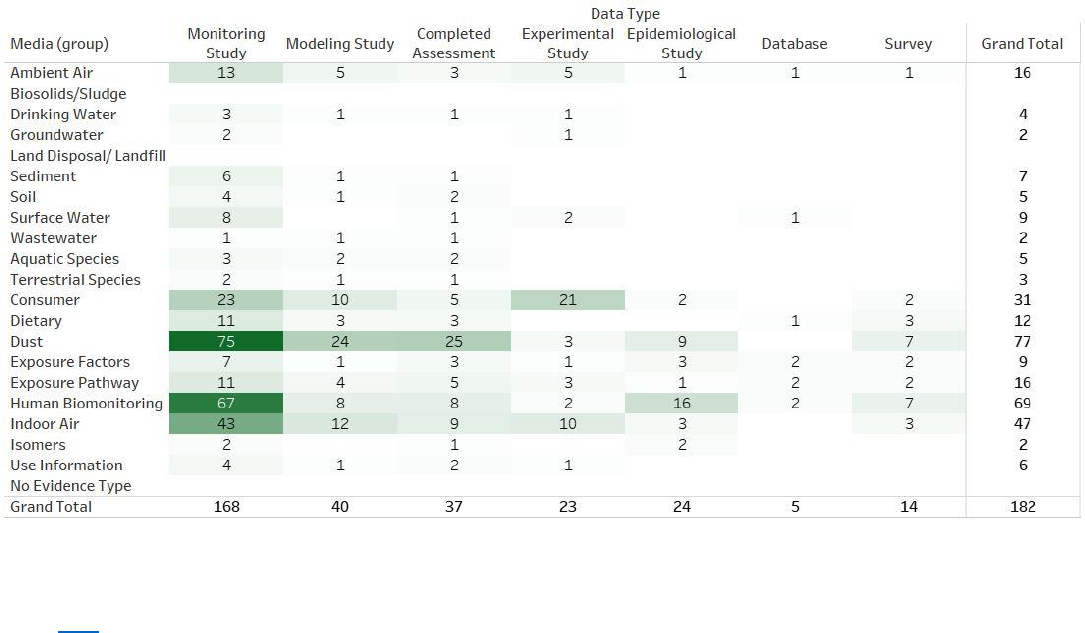
Figure 2-8. Peer-reviewed and Gray Literature Inventory Heat Map – Exposure Search Results
for TPP
Click here to view the interactive version for additional study details. The column totals, row totals, and
grand totals indicate total numbers of unique references, as some references may be included in multiple
cells. The various shades of color visually represent the number of relevant references identified by
exposure media or data type. The darker the color, the more references are available for a given
exposure media or data type. Data in this figure represent all references obtained from the publicly
available databases search (see Appendix A.1.2), and gray literature references search (see Appendix
A.3) that were included during full-text screening as of July 31, 2020. Additional data may be added the
interactive version as they become available.
22
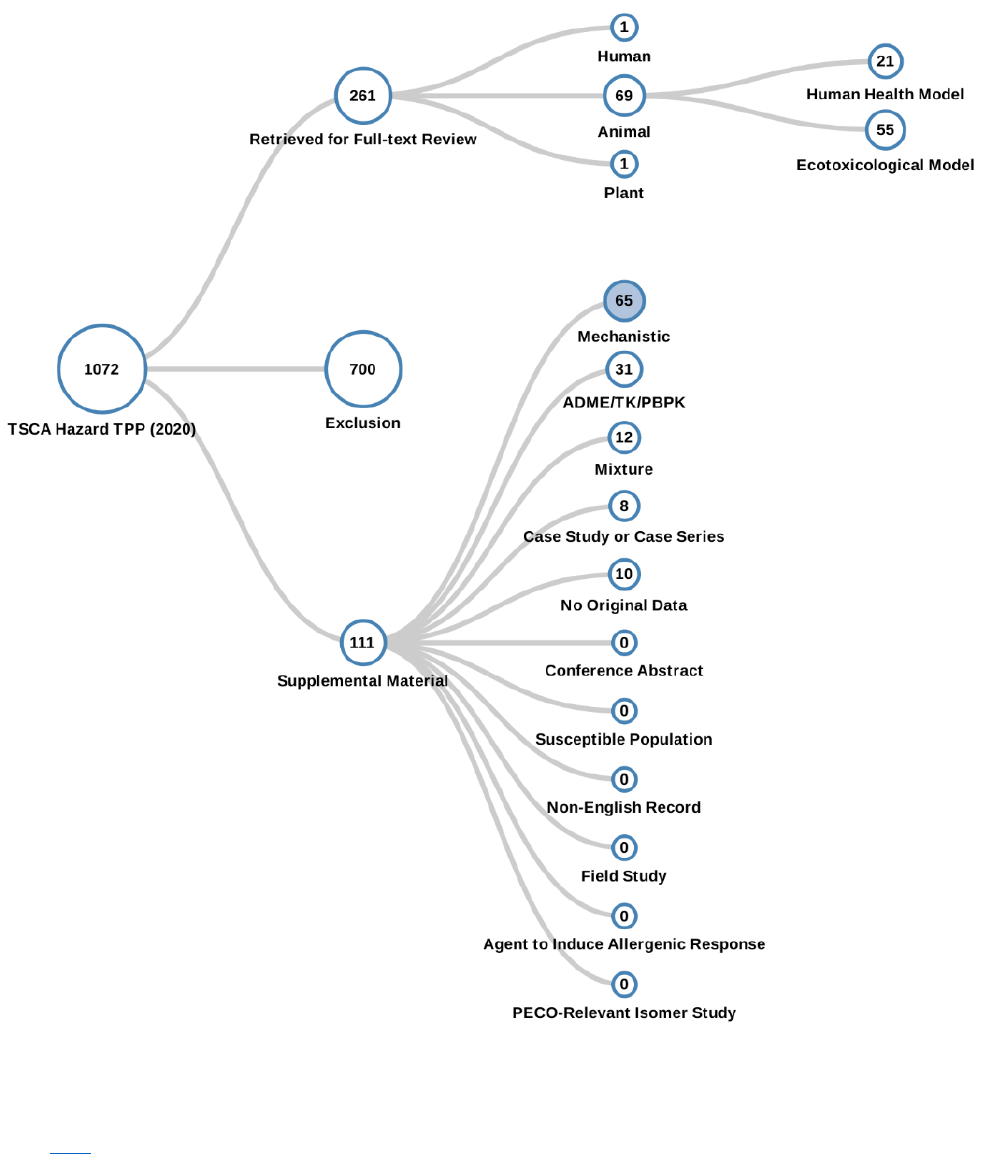
Figure 2-9. Peer-reviewed Literature Inventory Tree – Human Health and Environmental Hazard
Search Results for TPP
Click here to view the interactive literature inventory tree. Data in this figure represent references
obtained from the publicly available databases search (see Appendix A.1.2.) that were included during
full-text screening as of June 10, 2020. Additional data may be added to the interactive version as they
become available.
23
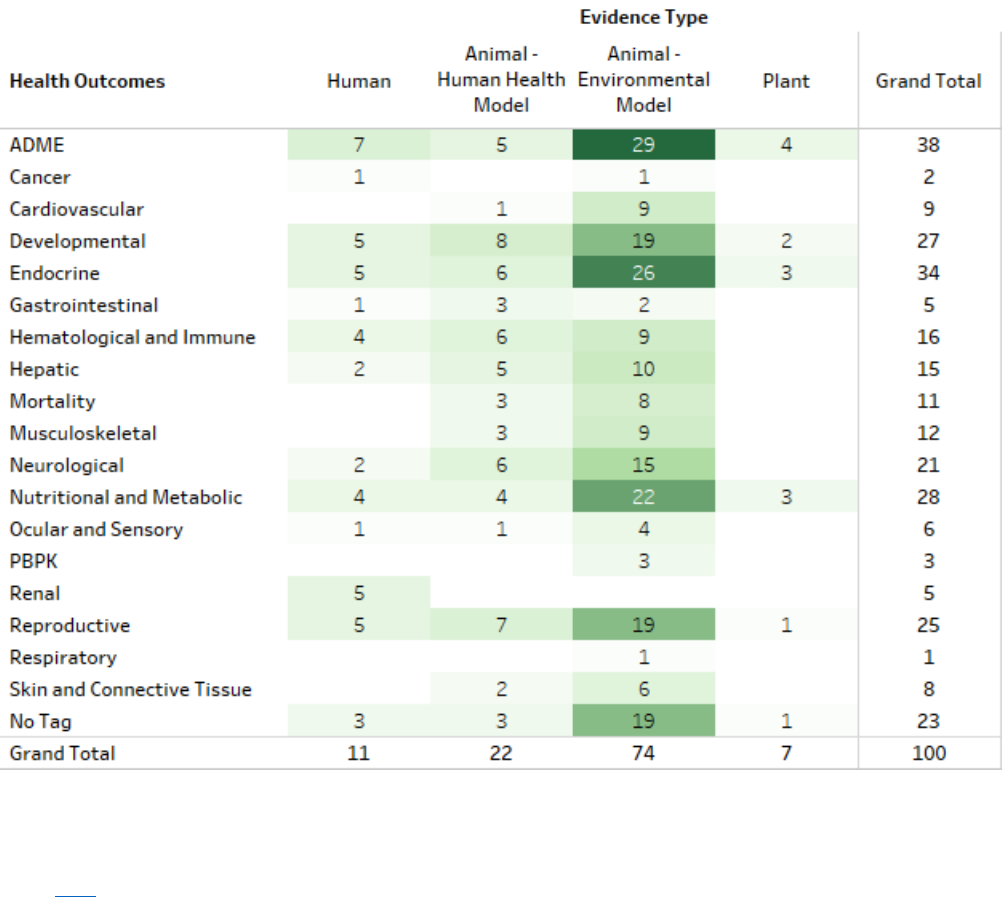
Figure 2-10. Peer-reviewed Literature Inventory Heat Map – Human Health and Environmental
Hazards Search Results for TPP
Click here to view the interactive version for additional study details. The numbers indicate the number
of studies with TIAB keywords related to a particular health outcome, not the number of studies that
observed an association with TPP. Evidence types were manually extracted, and Health Systems were
determined via machine learning. Therefore, the studies examining multiple Health Outcomes and
Evidence types, connections between health outcome, and evidence type may not be accurately
represented. If a study evaluated multiple health outcomes or included multiple populations or study
designs, it is shown here multiple times. Data in this figure represents references obtained from the
publicly available databases search (see Appendix A.1.2) that were included during full-text screening
as of June 10, 2020. Additional data may be added to the interactive version as they become available.
24
Search of TSCA Submissions
Table 2-1 presents the results of screening the titles of data sources and reports submitted to EPA under
various sections of TSCA. EPA screened a total of 295 submissions using PECO or similar statements
that identify inclusion/exclusion criteria specific to individual disciplines (see Table 2-1 for the list of
disciplines). The details about the criteria are presented in Appendix A.2.1. EPA identified 153
submissions that met the inclusion criteria in these statements and identified 130 submissions with
supplemental data.
3
EPA excluded 12 submissions because the reports were identified as one of the
following:
• Summary of other reports
• Draft of a published report that would be identified via peer literature searches
• Submission on a different chemical
• Data not relevant to any discipline
• Letter with no attached report
• Status report
• Notification of study initiation
Table 2-1. Results of Title Screening of Submissions to EPA under Various Sections of TSCA
Discipline
Included
a
Supplemental
a
Physical and Chemical Properties
26
0
Environmental Fate and Transport
70
0
Environmental and General Population
Exposure
14
0
Occupational Exposure/Release Information
13
0
Environmental Hazard
58
70
Human Health Hazard
37
74
a
Individual submissions may be relevant to multiple disciplines.
b
Included submissions may contain supplemental data for other disciplines, which will be identified at full-text review.
2.2 Conditions of Use
As described in the Proposed Designation of Triphenyl Phosphate (CASRN 115-86-6) as a High-
Priority Substance for Risk Evaluation (U.S. EPA, 2019d) EPA assembled information from the CDR
program to determine conditions of use
4
or significant changes in conditions of use of the chemical
substance. Once the 2020 CDR reporting period ends in November 2020, EPA utilize the most recent
CDR information. EPA also consulted a variety of other sources to identify uses of TPP, including the
following: published literature, company websites, and government and commercial trade databases and
publications. To identify formulated products containing TPP, EPA searched for safety data sheets
(SDS) using internet searches, EPA Chemical and Product Categories (CPCat) data, and other resources
in which SDSs could be found. SDSs were cross-checked with company websites to make sure that each
3
EPA may further consider some supplemental or excluded references depending on the reasons for tagging as supplemental
or excluded.
4
Conditions of use means the circumstances, as determined by the Administrator, under which a chemical substance is
intended, known, or reasonably foreseen to be manufactured, processed, distributed in commerce, used, or disposed of.
25
product SDS was current. In addition, EPA incorporated communications with companies, industry
groups, environmental organizations, and public comments to supplement the use information.
EPA identified and described the categories and subcategories of conditions of use that EPA plans to
include in the scope of the risk evaluation (Section 2.2.1; Table 2-2). The conditions of use included in
the scope are those reflected in the life cycle diagrams and conceptual models.
After gathering the reasonably available information related to the manufacture, processing, distribution
in commerce, use, and disposal of TPP, EPA identified those categories or subcategories of use activities
for TPP the Agency determined not to be conditions of use or will otherwise be excluded during
scoping. These categories and subcategories are described in Section 2.2.2.
Categories and Subcategories of Conditions of Use Included in the Scope of the
Risk Evaluation
Table 2-2 lists the conditions of use that are included in the scope of the risk evaluation.
Table 2-2. Categories and Subcategories of Conditions of Use Included in the Scope of the Risk
Evaluation
Life-Cycle Stage
a
Category
b
Subcategory
c
References
Manufacturing
Domestic
Manufacturing
Domestic Manufacturing
U.S. EPA (2019a)
Import
Import repackaging
U.S. EPA (2019a)
Processing
Incorporated into
formulation, mixture
or reaction product
Flame retardant used in all other
chemical product and preparation
manufacturing
U.S. EPA (2019a)
Flame retardant used in computer and
electronic product manufacturing
U.S. EPA (2019a)
Flame retardant used in plastics
material and resin manufacturing
U.S. EPA (2019a)
Plasticizer and flame retardant used in
plastic product manufacturing
U.S. EPA (2019a)
Flame retardant used in rubber
product manufacturing
U.S. EPA (2019a)
Flame retardant used in textiles,
apparel, and leather manufacturing
U.S. EPA (2019a)
Flame retardant used in utilities
U.S. EPA (2019a)
Paint additive and coating additive
used in paint and coating
manufacturing
U.S. EPA (2019a)
Flame retardant and plasticizer in all
other chemical product and
preparation manufacturing
U.S. EPA (2019a)
Flame retardant used in furniture and
related product manufacturing
U.S. EPA (2019a)
26
Life-Cycle Stage
a
Category
b
Subcategory
c
References
Plasticizer, additive and impurity in
adhesives, sealants and lubricants
Public Comment
EPA-HQ-OPPT-
2018-0458-0003
Flame retardant used in operational
fluids, maintenance fluids and
semisolids, reactive fluids, and solids
used in aerospace industry
Public Comment
EPA-HQ-OPPT-
2018-0458-0004
Flame retardant used in turbine engine
oils in aviation
Public Comment
EPA-HQ-OPPT-
2018-0458-0025
Flame retardant used in turbine engine
oils in non-aviation industries
Public Comment
EPA-HQ-OPPT-
2018-0458-0025
Flame retardant in lubricants and
greases
U.S. EPA (2019a)
Incorporated into
article
Flame retardant used in plastics
material and resin manufacturing
U.S. EPA (2019a)
Plasticizer used in plastics product
manufacturing
U.S. EPA (2019a)
Flame retardant used in furniture and
related product manufacturing
U.S. EPA (2019a)
Recycling
Recycling, e.g. electronics recycling
App. E 1.2.3
Distribution
Distribution in
commerce
Distribution in commerce
U.S. EPA (2019a)
Industrial/Commercial
Use
Paints and coatings
U.S. EPA (2019a)
Plastic and rubber products not
covered elsewhere
U.S. EPA (2019a)
Laboratory chemical
Public Comment
EPA-HQ-OPPT-
2018-0458-0034
Lubricants and greases
U.S. EPA (2019a)
Operational fluids, maintenance fluids
and semisolids, reactive fluids, and
solids used in aerospace industry
Public Comment
EPA-HQ-OPPT-
2018-0458-0004
Turbine engine oils used in aviation
Public Comment
EPA-HQ-OPPT-
2018-0458-0025
Turbine engine oils used in non-
aviation industries
Public Comment
EPA-HQ-OPPT-
2018-0458-0025
Electrical and electronic products
U.S. EPA (2019a)
Foam seating and bedding products
U.S. EPA (2019a)
Furniture and Furnishings not covered
elsewhere
U.S. EPA (2019a)
27
Life-Cycle Stage
a
Category
b
Subcategory
c
References
Consumer Use
Foam seating and bedding products
U.S. EPA (2019a)
Plastic and rubber products not
covered elsewhere
U.S. EPA (2019a)
Lubricants and greases
U.S. EPA (2019a)
Electrical and electronic products
U.S. EPA (2019a)
Disposal
Disposal
Disposal
a
Life Cycle Stage Use Definitions (40 CFR § 711.3)
‒ “Industrial use” means use at a site at which one or more chemicals or mixtures are manufactured (including
imported) or processed.
‒ “Commercial use” means the use of a chemical or a mixture containing a chemical (including as part of an article)
in a commercial enterprise providing saleable goods or services.
‒ “Consumer use” means the use of a chemical or a mixture containing a chemical (including as part of an article,
such as furniture or clothing) when sold to or made available to consumers for their use.
‒ Although EPA has identified both industrial and commercial uses here for purposes of distinguishing scenarios in
this document, the Agency interprets the authority over “any manner or method of commercial use” under TSCA
Section 6(a)(5) to reach both.
b
These categories of conditions of use appear in the Life Cycle Diagram, reflect CDR codes, and broadly represent
conditions of use of TPP in industrial and/or commercial settings and for consumer uses.
c
These subcategories reflect more specific conditions of use of TPP.
d.
In the final scope, EPA made the following changes to the conditions of use:
- One commenter recommended adding use as a laboratory chemical and EPA agreed. (EPA-HQ-OPPT-2019-0131-
0042)
- A commenter recommended amending the subcategory “Lubricants and Greases” because it is overly broad. (EPA-
HQ-OPPT-2018-0458-0025). EPA added two specific subcategories for aviation turbine oils.
- Use of TPP for photographic applications was removed as a condition of use based on a revised CDR entry.
Activities Excluded from the Scope of the Risk Evaluation
As explained in the final rule, Procedures for Chemical Risk Evaluation Under the Amended Toxic
Substances Control Act (U.S. EPA, 2017), TSCA Section 6(b)(4)(D) requires EPA to identify the
hazards, exposures, conditions of use, and the PESS the Administrator expects to consider in a risk
evaluation, suggesting that EPA may exclude certain activities that it determines to be conditions of use
on a case-by-case basis (82 FR 33726, 33729; July 20, 2017) (U.S. EPA, 2017). TSCA Section 3(4) also
grants EPA discretion to determine the circumstances that are appropriately considered to be conditions
of use for a particular chemical substance
5
. As a result, EPA does not plan to include in this scope or in
the risk evaluation the activities described below that the Agency does not consider to be conditions of
use or for which EPA is exercising discretionary authority provided by TSCA Section 6(b)(4)(D).
5
Chemical substance means any organic or inorganic substance of a particular molecular identity, including any combination
of such substances occurring in whole or in part as a result of a chemical reaction or occurring in nature, and any element or
uncombined radical. Chemical substance does not include (1) any mixture; (2) any pesticide (as defined in the Federal
Insecticide, Fungicide, and Rodenticide Act) when manufactured, processed, or distributed in commerce for use as a
pesticide; (3) tobacco or any tobacco product; (4) any source material, special nuclear material, or byproduct material (as
such terms are defined in the Atomic Energy Act of 1954 and regulations issued under such Act); (5) any article the sale of
which is subject to the tax imposed by Section 4181 of the Internal Revenue Code of 1954 (determined without regard to any
exemptions from such tax provided by Section 4182 or 4221 or any other provision of such Code), and; (6) any food, food
additive, drug, cosmetic, or device (as such terms are defined in Section 201 of the Federal Food, Drug, and Cosmetic Act)
when manufactured, processed, or distributed in commerce for use as a food, food additive, drug, cosmetic, or device (TSCA
§ 3(2)).

28
TSCA Section 3(2) also excludes from the definition of “chemical substance” “any food, food additive,
drug, cosmetic, or device (as such terms are defined in Section 201 of the Federal Food, Drug, and
Cosmetic Act [21 U.S.C. 321]) when manufactured, processed, or distributed in commerce for use as a
food, food additive, drug, cosmetic, or device” as well as “any pesticide (as defined in the Federal
Insecticide, Fungicide, and Rodenticide Act [7 U.S.C. 136 et seq.]) when manufactured, processed, or
distributed in commerce for use as a pesticide.” EPA has determined that the following uses of TPP are
non-TSCA uses:
EPA is aware of information reporting TPP in the manufacture and use of nail polish (EWG, 2019)and
in flea and tick collars (Central Garden & Pet, 2017). These activities are not “conditions of use”
(defined in TSCA § 3(4) as circumstances associated with “a chemical substance,” as defined in TSCA §
3(2)). TSCA defines “chemical substance” to exclude cosmetics, which are covered under the Federal
Food, Drug and Cosmetics Act (FFDCA), 21 U.S.C. § 321, and pesticides, which are covered under
EPA’s Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), 7 U.S.C. § 136 et seq. Therefore,
the uses of TPP in cosmetics and pesticides are outside the scope of the definition of chemical substance
as regulated by TSCA and EPA does not plan to consider those activities in the risk evaluation.
Production Volume
As reported to EPA during the 2016 CDR reporting period and described here as a range to protect
production volumes that were claimed as confidential business information (CBI), total production
volume of TPP in 2015 was between 1 million and 10 million pounds (U.S. EPA, 2020a). EPA also uses
pre-2015 CDR production volume information, as detailed in the Proposed Designation of Triphenyl
Phosphate (CASRN 115-86-6) as a High-Priority Substance for Risk Evaluation (U.S. EPA, 2019d) and
will include more recent production volume information from the 2020 CDR reporting period in the risk
evaluation to support the exposure assessment.
Overview of Conditions of Use and Lifecycle Diagram
Figure 2-11 provides the lifecycle diagram for TPP. The life cycle diagram is a graphical representation
of the various life stages of the industrial, commercial and consumer use categories included within the
scope of the risk evaluation. The information in the life cycle diagram is grouped according to the CDR
processing codes and use categories (including functional use codes for industrial uses and product
categories for industrial, commercial and consumer uses). Appendix E contains more detailed
descriptions (e.g., process descriptions, worker activities, process flow diagrams) for each manufacture,
processing, distribution in commerce, use and disposal category.
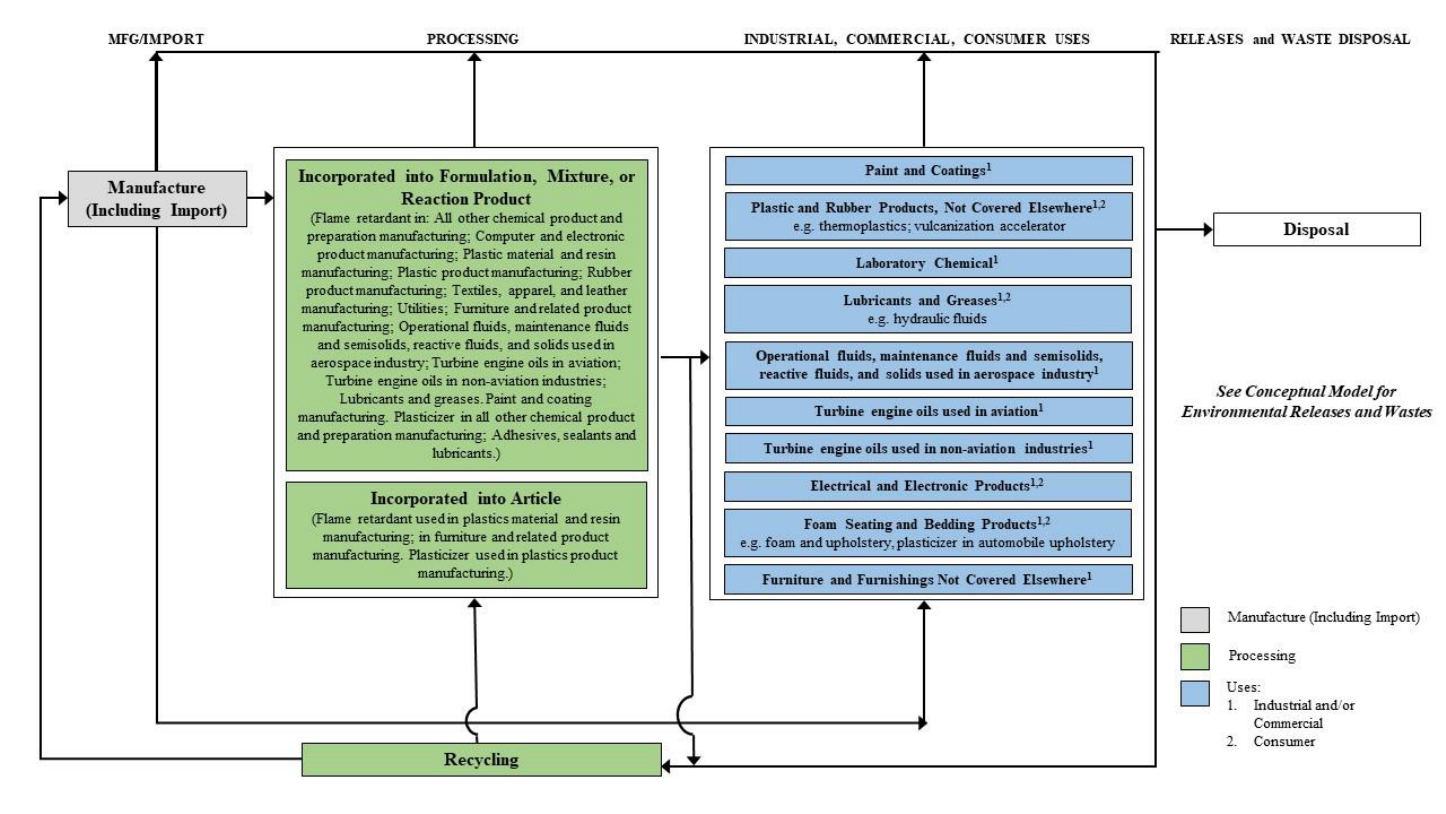
29
Figure 2-11. TPP Life Cycle Diagram
30
2.3 Exposures
For TSCA exposure assessments, EPA plans to analyze human and environmental exposures and
releases to the environment resulting from the conditions of use within the scope of the risk evaluation
of TPP. In this section, the physical and chemical properties, environmental fate and transport properties
and releases to the environment are described in addition to potential human and environmental
exposures from TSCA conditions of use and from other possible or known sources. Release pathways
and routes will be described in Section 2.6 to characterize the relationship or connection between the
conditions of use of the chemical and the exposure to human receptors, including PESS, and
environmental receptors. EPA plans to consider where relevant, the duration, intensity (concentration),
frequency and number of exposures in characterizing exposures to TPP.
Physical and Chemical Properties
Consideration of physical and chemical properties is essential for a thorough understanding or prediction
of environmental fate (i.e., transport and transformation) and the eventual environmental concentrations.
It can also inform the hazard assessment. Table 2-3 summarizes the physical and chemical property
values preliminarily selected for use in the risk evaluation from among the range of reported values
collected as of June 2020. This information differs from that presented in the Proposed Designation of
Triphenyl Phosphate (CASRN 115-86-6) as a High-Priority Substance for Risk Evaluation (U.S. EPA,
2019d) and may be updated as EPA continues to evaluate and integrate additional information through
systematic review methods. Figure 2-12 summarizes the distribution of reported values for eight
physical and chemical properties routinely used in existing chemical risk evaluations. Appendix B
presents summary statistics for reported physical and chemical property values. All physical and
chemical property values that were extracted and evaluated as of June 2020 are presented in the
supplemental file Data Extraction and Data Evaluation Tables for Physical and Chemical Property
Studies (EPA-HQ-OPPT-2018-0458).
Table 2-3. Physical and Chemical Properties of TPP
Property or Endpoint
Value
a
Reference
Data Quality Rating
Molecular formula
C
18
H
15
O
4
P
1
NA
NA
Molecular weight
326.29 g/mol
NA
NA
Physical state
Solid crystals or prisms
Rumble J. R. (2018)
High
Physical properties
Colorless, crystalline
powder; odorless
NLM (2018)
High
Melting point
49.39°C
Rumble J. R. (2018)
High
Boiling point
414°C
U.S. EPA (2019c)
High
Density
1.2055 g/cm
3
at 50°C
Rumble J. R. (2018)
High
Vapor pressure
6.28×10
-6
mm Hg
U.S. EPA (2019c)
High
Vapor density
1.19 (air = 1)
NLM (2018)
High
Water solubility
1.9 mg/L at 25°C
NLM (2018)
High
31
Property or Endpoint
Value
a
Reference
Data Quality Rating
Log Octanol/water partition
coefficient (Log K
ow
)
4.59
NLM (2018)
High
Henry’s Law constant
1.42×10
-6
atm·m
3
/mol
(Calculated from
VP/WS)
U.S. EPA (2012b)
High
Flash point
220°C
RSC (2019)
High
Auto flammability
Not available
Viscosity
Not available
Refractive index
1.550
NLM (2018)
High
Dielectric constant
Not available
a
Measured unless otherwise noted.
NA = Not applicable
Figure 2-12 displays a summary of the data collected as of June 2020 for eight physical and chemical
values routinely used in TSCA existing chemical risk evaluations. The box and whisker plots for each
endpoint illustrate the mean (average, indicated by the blue diamond) and the 10
th
, 25
th
, 50
th
(median),
75
th
, and 90
th
percentiles. All individual data points are indicated by black squares, and values
preliminarily selected for use in the risk evaluation is overlaid (indicated by the orange circle) to provide
context for where it lies within the distribution of the dataset. The number of unique primary data
sources is indicated below each box and whisker plot. If multiple sources presented equivalent values
and cited the same primary source, only one of those was included in the statistical calculations. As a
result, the number of sources listed in Figure 2-12 may differ from the total number of data sources
presented in Figure 2-2. Where no data could be identified through systematic review, text appears to
clearly demonstrate the gap for the endpoint.
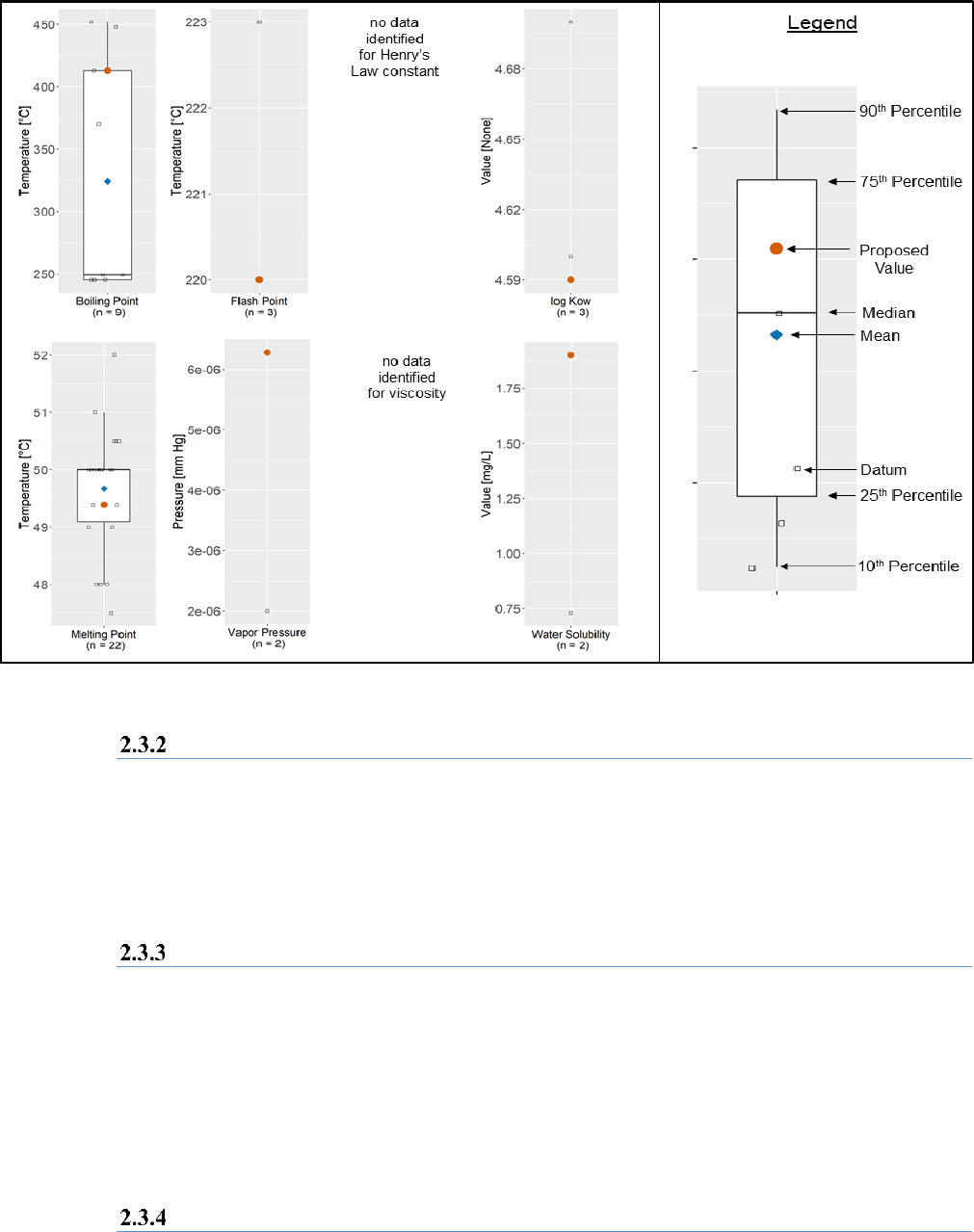
32
Figure 2-12. Box and Whisker Plots of Reported Physical and Chemical Property Values
Environmental Fate and Transport
Understanding of environmental fate and transport processes assists in the determination of the specific
exposure pathways and potential human and environmental receptors that need to be assessed in the risk
evaluation for TPP. EPA plans to use the environmental fate characteristics described in Appendix C to
support the development of the risk evaluation for TPP. The values for the environmental fate properties
may be updated as EPA evaluates and integrates additional information into the risk evaluation through
systematic review methods.
Releases to the Environment
Releases to the environment from conditions of use are a component of potential exposure and may be
derived from reported data that are obtained through direct measurement, calculations based on
empirical data and/or assumptions and models.
TPP is not reported to the Toxics Release Inventory (TRI). There may be releases of TPP from industrial
sites to wastewater treatment plants (WWTP), surface water, air and landfill. Articles that contain TPP
may release TPP to the environment during use or through recycling and disposal. EPA plans to review
this data in conducting the exposure assessment component of the risk evaluation for TPP.
Environmental Exposures
The manufacturing, processing, distribution, use and disposal of TPP can result in releases to the
environment and exposure to aquatic and terrestrial receptors (biota). Environmental exposures to biota

33
are informed by releases into the environment, overall persistence, degradation, bioaccumulation and
partitioning across different media. Concentrations of chemical substances in biota provide evidence of
exposure. EPA plans to review available environmental exposure data in biota in the risk evaluation.
Monitoring data were identified in EPA’s search for reasonably available information on environmental
exposures in biota to inform development of the environmental exposure assessment for TPP.
EPA plans to review available environmental monitoring data for TPP. TPP was detected in wastewater
effluent, landfill leachate, sediment, soil, ambient air, as well as in fish (including shellfish) and dolphins
(U.S. EPA, 2015b; UK Environment Agency, 2009; OECD, 2002). According to the USGS Monitoring
Data – National Water Quality Monitoring Council, TPP exists in various organisms (USGS, 1991g).
Occupational Exposures
EPA plans to evaluate worker activities where there is a potential for exposure under the various
conditions of use (manufacturing, processing, industrial/commercial uses, and disposal) described in
Section 2.2. In addition, EPA plans to evaluate exposure to occupational non-users (ONUs), i.e.,
workers who do not directly handle the chemical but perform work in an area where the chemical is
present. EPA also plans to consider the effect(s) that engineering controls (EC) and/or personal
protective equipment (PPE) have on occupational exposure levels as part of the risk evaluation.
EPA plans to evaluate potential exposures from the processing of TPP as it is incorporated into
formulations and products. TPP is used as an additive flame retardant. In general, EPA plans evaluate
the potential for exposure from additive flame retardants due to blooming and release from article
components during their manufacture and industrial/commercial use. TPP is also used as a component of
liquid products; including, but not limited to paints, coatings, lubricants and greases.
Examples of worker activities associated with the conditions of use within the scope of the risk
evaluation for TPP that EPA may analyze include, but are not limited to:
• Unloading and transferring TPP to and from storage containers to process vessels during
manufacturing, processing and use;
• Handling and disposing of waste containing TPP;
• Cleaning and maintaining equipment;
• Sampling chemicals, formulations or products containing TPP for quality control;
• Repackaging chemicals, formulations or products containing TPP during manufacturing,
processing, use and recycling; and
• Performing other work activities in or near areas where TPP is used.
TPP is a solid with a vapor pressure of approximately 6.3×10-6 mm Hg at 25 ºC/77 ºF (see Section
2.3.1). EPA anticipates inhalation of mist, dust, and other respirable particles as an exposure pathway for
workers and occupational non-users during the manufacture, processing, and commercial/industrial use
of various products containing TPP (e.g., particulate generated during manufacture and handling of foam
and plastics and incorporation of foam and plastics into finished products, and mist generated during
application to textiles and application of paints and coatings).
EPA generally does not evaluate occupational exposures through the oral route. Workers and ONUs
may inadvertently ingest inhaled particles that deposit in the upper respiratory tract. In addition, workers
may transfer chemicals from their hands to their mouths. The frequency and significance of this
exposure route are dependent on several factors including the physical and chemical properties of the
substance during expected worker activities, workers’ awareness of the chemical hazards, the visibility
34
of the chemicals on the hands while working, workplace training and practices, and personal hygiene
that is difficult to predict (Cherrie et al., 2006). EPA will consider the relevance of this exposure route
on a case-by-case basis, taking into consideration the aforementioned factors and any reasonably
available information, and may assess oral exposure for workers for certain COUs and worker activities
where warranted. For certain conditions of use of TPP, EPA plans to consider inhalation exposure to
dust/particulates for workers and ONUs. As inhalation exposure to dust/particulates may occur, EPA
plans to consider potential exposure for particulates that deposit in the upper respiratory tract from
inhalation exposure and may be ingested via the oral route
TPP has an Occupational Safety and Health Administration (OSHA) Permissible Exposure Limit (PEL).
The PEL is 3 milligrams (mg)/cubic meter (m
3
) over an 8-hour workday, time weighted average (TWA).
The American Conference of Governmental Industrial Hygienists (ACGIH) set the Threshold Limit
Value (TLV) at 3 ppm TWA (OSHA, 2019). Also, the OSHA Permissible Exposure Limit (PEL) for
Particulates Not Otherwise Regulated (PNOR) (15 mg/m
3
) (29 CFR 1910.1000) may be applicable if
particulate matter is generated during industrial operations. This chemical also has a National Institute
for Occupational Safety and Health (NIOSH) Recommended Exposure Limit (REL) of 3 mg/m
3
TWA
(NIOSH, 2019) and an Immediately Dangerous to Life or Health (IDLH) value of 1,000 mg/m
3
(NIOSH, 2016).
EPA plans to evaluate dermal exposure to workers from contact with solids during packaging and
repackaging operations at manufacturing and import sites when TPP is handled as a dry powder. EPA
also anticipates dermal exposure to liquid if TPP is formulated with liquid chemical and handled as a
liquid. Dermal exposure by ONU is not expected for these conditions of use as they are not expected to
directly handle the chemical.
Consumer Exposures
According to CDR, TPP is used in consumer products used in indoor environments, including foam
seating and bedding products, plastic and rubber products, and (U.S. EPA, 2019a). The 2012 CDR also
reported the use of TPP in electrical and electronic products (U.S. EPA, 2019a). Several of these
products have the potential to be mouthed by children. In addition, consumer handling of the disposal on
TPP containing materials can lead to consumer and bystander exposures. The main exposure routes for
these uses where consumers interact with products and articles containing TPP are dermal, inhalation,
and dust ingestion, including children’s mouthing of articles (e.g., plastics, textiles, wood products)
containing TPP. Based on these potential sources and pathways of exposure, EPA plans to analyze oral,
dermal and inhalation routes of exposure to consumers, and the inhalation route for bystanders that may
result from the conditions of use of TPP.
General Population Exposures
Releases of TPP from certain conditions of use, such as manufacturing, processing, or disposal
activities, may result in general population exposures. TPP was detected in surface water, ground water,
soil, ambient air, indoor air, indoor dust, as well as in fish (including shellfish) (U.S. EPA, 2015b; UK
Environment Agency, 2009; OECD, 2002; USGS, 1991a, b, c, d, e, f, g). EPA plans to evaluate the
reasonably available literature for the presence of TPP in drinking water, ground water, ambient air,
indoor air, fish, human breast milk, and dust and soil, which may be mouthed or ingested. The general
population pathways in the scope of this evaluation are described in Sections 2.6.3 and 2.7.2.5.
35
2.4 Hazards (Effects)
Environmental Hazards
EPA considered reasonably available information (e.g., federal and international government chemical
assessments) on TPP as well as public comments received on the Proposed Designation of Triphenyl
Phosphate (CASRN 115-86-6) as a High-Priority Substance for Risk Evaluation (U.S. EPA, 2019d), and
draft scope for TPP (U.S. EPA, 2020c) to identify potential environmental hazards. During
prioritization, EPA identified environmental hazard effects for aquatic and terrestrial organisms.
Since prioritization, EPA applied automated techniques during the data screening phase of systematic
review to identify the following potential environmental hazards and related information that may be
considered for the risk evaluation (as explained in Appendix A): ADME, PBPK, cancer, cardiovascular,
developmental, endocrine, gastrointestinal, hematological and immune, hepatic, mortality, musculo-
skeletal, neurological, nutritional and metabolic, ocular and sensory, reproductive, respiratory and skin
and connective tissue (Figure 2-10). A summary of references identified during the screening step of
systematic review is included in the interactive literature inventory trees (Figure 2-9). As EPA continues
to evaluate reasonably available and relevant hazard information identified through systematic review,
EPA may update the list of potential hazard effects to be analyzed in the risk evaluation.
Human Health Hazards
EPA considered reasonably available information (e.g., federal and international government chemical
assessments) on TPP as well as public comments on the Proposed Designation of Triphenyl Phosphate
(CASRN 115-86-6) as a High-Priority Substance for Risk Evaluation (U.S. EPA, 2019d), and draft
scope for TPP (U.S. EPA, 2020c) to identify potential human health hazards. During prioritization, EPA
identified the following potential human health hazards and related information: repeated dose,
developmental and irritation and corrosion.
Since prioritization, EPA applied automated techniques during the data screening phase of systematic
review to identify the following additional potential human health hazards and related information that
may be considered for the risk evaluation (as explained in Appendix A): ADME, cancer, cardiovascular,
endocrine, gastrointestinal, hematological and immune, hepatic, mortality, musculoskeletal,
neurological, nutritional and metabolic, ocular and sensory, renal, reproductive and skin and connective
tissue (Figure 2-10). A summary of references identified during the screening step of systematic review
is included in the interactive literature inventory trees (Figure 2-9). As EPA continues to evaluate
reasonably available and relevant hazard information identified through systematic review, EPA may
update the list of potential hazard effects to be analyzed in the risk evaluation.
2.5 Potentially Exposed or Susceptible Subpopulations
TSCA § 6(b)(4) requires EPA to determine whether a chemical substance presents an unreasonable risk
to “a potentially exposed or susceptible subpopulation identified as relevant to the risk evaluation.”
TSCA §3(12) states that “the term ‘potentially exposed or susceptible subpopulation’ means a group of
individuals within the general population identified by the Administrator who, due to either greater
susceptibility or greater exposure, may be at greater risk than the general population for adverse health
effects from exposure to a chemical substance or mixture, such as infants, children, pregnant women,
workers, or the elderly.” General population is "the total of individuals inhabiting an area or making up a
whole group” and refers here to the U.S. general population (U.S. EPA, 2011a).

36
EPA identified the following PESS based on CDR information and studies reporting developmental and
reproductive effects: children, women of reproductive age (e.g., pregnant women), lactating females,
workers, including ONUs and users, and consumers, including users and bystanders (U.S. EPA, 2019b).
EPA plans to evaluate these PESS in the risk evaluation. Following further evaluation of the reasonably
available information, EPA may evaluate PESS in the general population as they relate to fence line
communities.
In developing exposure scenarios, EPA plans to analyze reasonably available data to ascertain whether
some human receptor groups may be exposed via exposure pathways that may be distinct to a particular
subpopulation or life stage (e.g., children’s crawling, mouthing or hand-to-mouth behaviors, ingestion of
breast milk) and whether some human receptor groups may have higher exposure via identified
pathways of exposure due to unique characteristics (e.g., activities, duration or location of exposure)
when compared with the general population (U.S. EPA, 2006b). Likewise, EPA plans to evaluate
reasonably available human health hazard information to ascertain whether some human receptor groups
may have greater susceptibility than the general population to the chemical’s hazard(s). Based on these
analyses, EPA may update the list of PESS in the risk evaluation.
2.6 Conceptual Models
In this section, EPA presents the conceptual models describing the identified exposures (pathways and
routes), receptors and hazards associated with the conditions of use of TPP. Pathways and routes of
exposure associated with workers and ONUs are described in Section 2.6.1, and pathways and routes of
exposure associated with consumers are described in Section 2.6.2. Pathways and routes of exposure
associated with environmental releases and wastes are depicted in the conceptual model shown in
Section 2.6.3.
Conceptual Model for Industrial and Commercial Activities and Uses
Figure 2-13 illustrates the conceptual model for the pathways of exposure from industrial and
commercial activities and uses of TPP that EPA plans to evaluate in the risk evaluation. There is
potential for exposure to workers and ONUs via inhalation/oral routes and exposures to workers via
dermal routes. Dermal exposure to TPP in both liquid and solid form is expected, as TPP can be used/
transported in solid form or suspended in solution. Inhalation exposure to dust is expected to be a
significant exposure pathway. Additionally, potential inhalation exposure to TPP in mist form is
expected for certain conditions of use. EPA plans to evaluate activities resulting in exposures associated
with distribution in commerce (e.g., loading, unloading) throughout the various lifecycle stages and
conditions of use (e.g., manufacturing, processing, industrial use, commercial use, and disposal) rather
than a single distribution scenario.
For each condition of use identified in Table 2-2, a determination was made as to whether or not EPA
plans to evaluate each combination of exposure pathway, route, and receptor will be assessed in the risk
evaluation. The supporting rationale are presented in Appendix F.
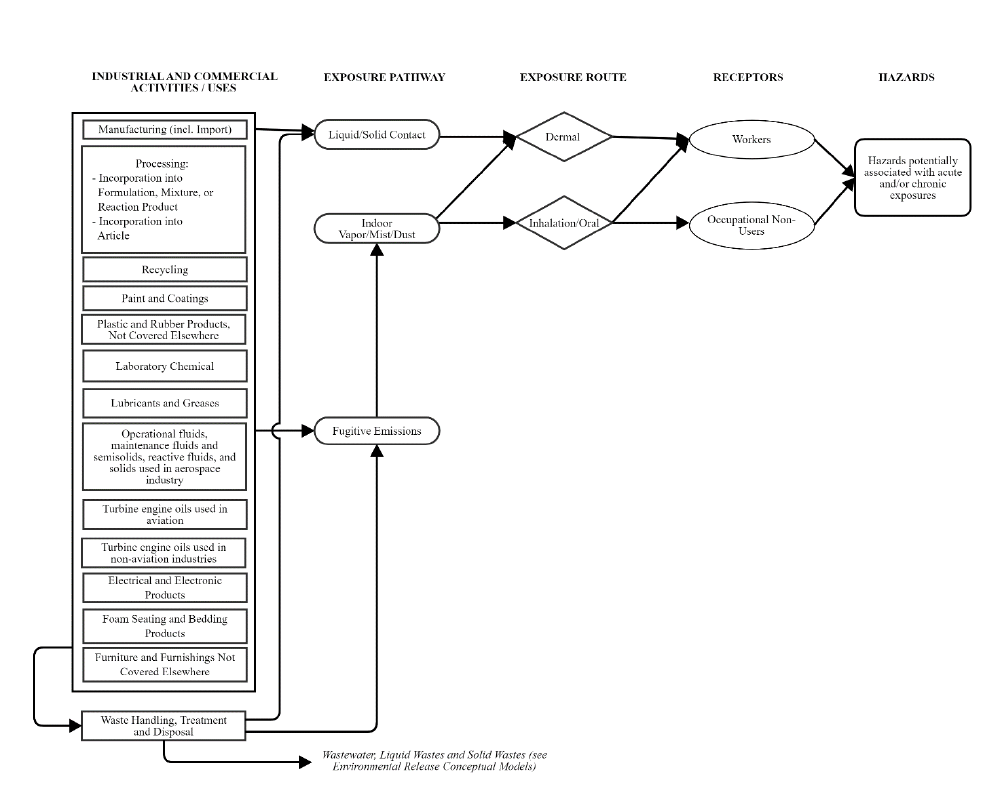
37
Figure 2-13. TPP Conceptual Model for Industrial and Commercial Activities and Uses: Worker and ONU Exposures and Hazards
The conceptual model presents the exposure pathways, exposure routes, and hazards to human receptors from industrial and commercial
activities and uses of TPP.

38
Conceptual Model for Consumer Activities and Uses
The conceptual model in Figure 2-14 presents the exposure pathways, exposure routes and hazards to
human receptors from consumer activities and uses of TPP that EPA plans to include in the risk
evaluation. Inhalation is expected to be a route of exposure during use of consumer products and EPA
plans to evaluate inhalation exposures to TPP in vapor, mist, and dust for consumers and bystanders.
Consumer oral exposures may also result from direct contact with mist and powders or dust containing
TPP during use. Dermal exposures may result from liquids, and mist containing TPP. Bystanders are not
expected to have direct dermal or oral contact to TPP containing products. The supporting rationale for
consumer pathways considered for TPP are included in Appendix G.
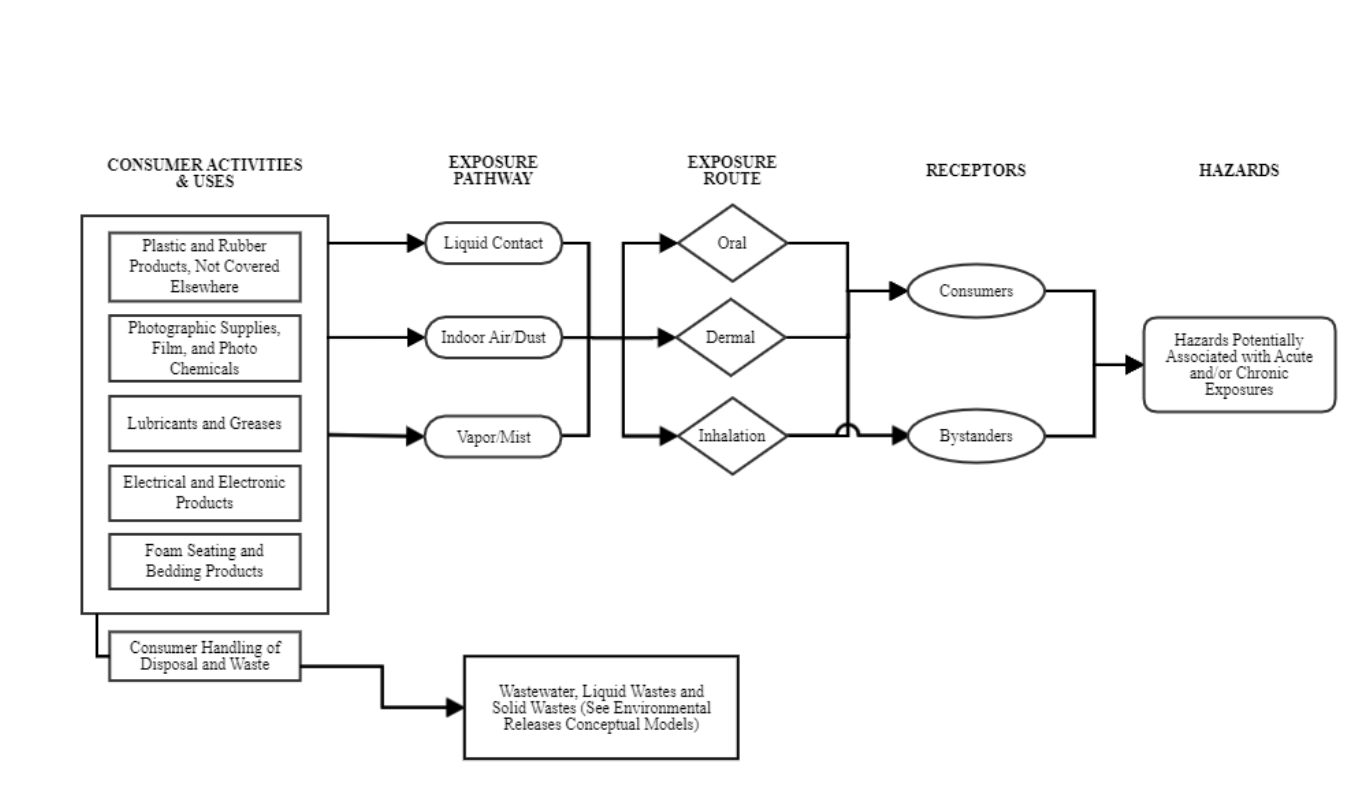
39
Figure 2-14. TPP Conceptual Model for Consumer Activities and Uses: Consumer Exposures and Hazards
The conceptual model presents the exposure pathways, exposure routes and hazards to human receptors from consumer activities and uses of
TPP.

40
Conceptual Model for Environmental Releases and Wastes: Potential
Exposures and Hazards
Figure 2-15 presents the exposure pathways, exposure routes, and hazards to general population and
environmental receptors for releases and waste streams associated with environmental releases of TPP.
EPA plans to evaluate pathways and routes of exposures to receptors (e.g., general population, aquatic,
terrestrial species) that may occur from industrial and/or commercial uses, releases to air, water or land,
including biosolids and soil, and other conditions of use. EPA expects humans to be exposed to TPP
from air emissions via inhalation as well as from water, liquid, and solid waste releases and orally via
drinking water, surface water, fish and soil ingestion, and dermally from contact with drinking water,
surface water, groundwater and soil. The supporting rationale for general population and environmental
pathways considered for TPP are included in Appendix H.
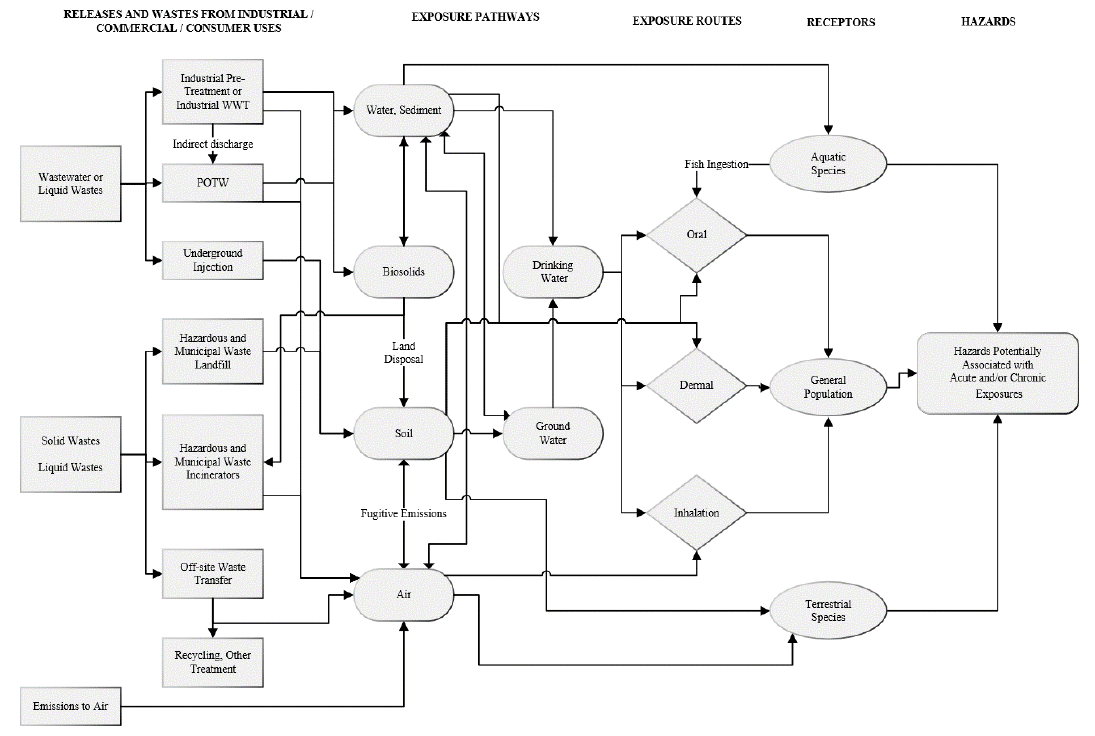
41
Figure 2-15. TPP Conceptual Model for Environmental Releases and Wastes: Environmental and General Population Exposure and
Hazards
The conceptual model presents the exposure pathways, exposure routes and hazards to human and environmental receptors from releases and
wastes from industrial, commercial, and consumer uses of TPP.
a) Industrial wastewater or liquid wastes may be treated on-site and then released to surface water (direct discharge), or pre-treated and released to
Publicly Owned Treatment Works (POTW) (indirect discharge). For consumer uses, such wastes may be released directly to POTW. Drinking water
will undergo further treatment in drinking water treatment plant. Ground water may also be a source of drinking water.
b) Receptors include PESS (see Section 2.5).

42
2.7 Analysis Plan
The analysis plan is based on EPA’s knowledge of TPP resulting from the full-text screening of
reasonably available information as described in Section 2.1. EPA encourages submission of additional
existing data, such as full study reports or workplace monitoring from industry sources, that may be
relevant to EPA’s evaluation of conditions of use, exposures, hazards and PESS during risk evaluation.
As discussed in the Application of Systematic Review in TSCA Risk Evaluations document (U.S. EPA,
2018a), targeted supplemental searches during the analysis phase may be necessary to identify additional
information (e.g., commercial mixtures) for the risk evaluation of TPP. For any additional data needs
identified during the risk evaluation, EPA may use the Agency’s TSCA authorities under Sections 4, 8
or 11, as appropriate.
Physical and Chemical Properties and Environmental Fate
EPA plans to analyze the physical and chemical properties and environmental fate and transport of TPP
as follows:
1) Review reasonably available measured or estimated physical and chemical and
environmental fate endpoint data collected using systematic review procedures and, where
reasonably available, environmental assessments conducted by other regulatory agencies.
EPA plans evaluate data and information collected through the systematic review methods and
public comments about the physical and chemical properties (Appendix B) and fate endpoints
(Appendix C), some of which appeared in the Proposed Designation of Triphenyl Phosphate
(CASRN 115-86-6) as a High-Priority Substance for Risk Evaluation (U.S. EPA, 2019d). All
sources cited in EPA’s analysis will be evaluated according to the procedures and metrics
described in the Application of Systematic Review in TSCA Risk Evaluations (U.S. EPA, 2018a).
Where the systematic review process does not identify experimentally measured chemical
property values of sufficiently high quality, testing will be requested under the TSCA Section 4
authority, or values will be estimated using chemical parameter estimation models as
appropriate. Model-estimated fate properties will be reviewed for applicability and quality
2) Using measured data and/or modeling, determine the influence of physical and chemical
properties and environmental fate endpoints (e.g., persistence, bioaccumulation,
partitioning, transport) on exposure pathways and routes of exposure to human and
environmental receptors.
Measured data and, where necessary, model predictions of physical and chemical properties and
environmental fate endpoints will be used to characterize the persistence and movement of TPP
within and across environmental media. The fate endpoints of interest include volatilization,
sorption to organic matter in soil and sediments, water solubility, aqueous and atmospheric
photolysis rates, aerobic and anaerobic biodegradation rates, and potential bioconcentration and
bioaccumulation. These endpoints will be used in exposure calculations.

43
3) Conduct a weight of the scientific evidence evaluation of physical and chemical and
environmental fate data, including qualitative and quantitative sources of information.
During risk evaluation, EPA plans to evaluate and integrate the physical and chemical and
environmental fate evidence identified in the literature inventory using the methods described in
the Application of Systematic Review in TSCA Risk Evaluations (U.S. EPA, 2018a).
Exposure
EPA plans to analyze exposure levels for indoor air, ambient air, surface water, sediment, soil, aquatic
biota, and terrestrial biota associated to exposure to TPP. Based on its physical and chemical properties,
expected sources, and transport and transformation within the outdoor and indoor environment, TPP is
more likely to be present in some of these media and less likely to be present in others. EPA has not yet
determined the exposure levels in these media. Exposure level(s) can be characterized through a
combination of reasonably available monitoring data and estimated exposure levels from modeling
approaches. Exposure scenarios are combinations of sources (uses), exposure pathways, and exposed
receptors. Draft exposure scenarios corresponding to various conditions of use for TPP are presented in
Appendix F, Appendix G and Appendix H. EPA plans to analyze scenario-specific exposures.
2.7.2.1 Environmental Releases
EPA plans to analyze releases to environmental media as follows:
1) Review reasonably available published literature and other reasonably available
information on processes and activities associated with the conditions of use to analyze the
types of releases and wastes generated.
EPA has reviewed some sources containing information on processes and activities resulting in
releases, and the information found is described in Appendix E. EPA plans to review additional
sources identified. Potential sources of environmental release data are summarized in Table 2-4:
Table 2-4. Categories and Sources of Environmental Release Data
U.S. EPA Generic Scenarios
OECD Emission Scenario Documents
UK Environmental Risk Evaluation Report
Discharge Monitoring Report (DMR) surface water discharge data for TPP from NPDES-
permitted facilities
2) Review reasonably available chemical-specific release data, including measured or
estimated release data (e.g., data from risk assessments by other environmental agencies).
EPA plans to continue to review relevant data sources during the risk evaluation. EPA will
continue to consider additional reasonably available information and will evaluate it during
development of the risk evaluation. EPA plans to match identified data to applicable conditions
of use and identify data gaps where no data are found for particular conditions of use. EPA plans
to attempt to address data gaps identified as described in #3 and #4 below by considering
potential surrogate data and models.
Additionally, for conditions of use where no measured data on releases are reasonably available,
EPA may use a variety of methods including release estimation approaches and assumptions in
the Chemical Screening Tool for Exposures and Environmental Releases (ChemSTEER) (U.S.
EPA, 2013).

44
3) Review reasonably available measured or estimated release data for surrogate chemicals
that have similar uses and physical properties.
EPA plans to review literature sources identified and if surrogate data are found, these data will
be matched to applicable conditions of use for potentially filling data gaps.
4) Review reasonably available data that may be used in developing, adapting or applying
exposure models to the particular risk evaluation.
This item will be performed after completion of #2 and #3 above. EPA plans to evaluate relevant
data to determine whether the data can be used to develop, adapt or apply models for specific
conditions of use (and corresponding release scenarios). EPA has identified information from
various EPA statutes (including, for example, regulatory limits, reporting thresholds or disposal
requirements) that may be relevant to release estimation. EPA plans to further consider relevant
regulatory requirements in estimating releases during risk evaluation.
5) Review and determine applicability of OECD Emission Scenario Documents (ESDs) and
EPA Generic Scenarios to estimation of environmental releases.
EPA has identified potentially relevant OECD Emission Scenario Documents (ESDs) and EPA
Generic Scenarios (GS) that correspond to some conditions of use; for example, the 2009 ESD
on Plastics Additives and the 2011 ESD on the Chemical Industry may be useful. EPA plans to
critically review these generic scenarios and ESDs to determine their applicability to the
conditions of use.
EPA Generic Scenarios are available at the following: https://www.epa.gov/tsca-screening-
tools/chemsteer-chemical-screening-tool-exposures-and-environmental-releases
Generic Scenarios that contain information that may be related to the potential uses of TPP
include, but are not limited to:
• EPA’s Additives in Plastics Processing (Compounding) – Draft Generic Scenario for
Estimating Occupational Exposures and Environmental Releases (May 2004) ;EPA’s Spray
Coatings in the Furniture Industry - Generic Scenario for Estimating Occupational
Exposures and Environmental Releases (April 2004);
• EPA’s Leather Dyeing - Generic Scenario for Estimating Occupational Exposures and
Environmental Releases (September 2000);
• EPA’s Fabric Finishing – Draft Generic Scenario for Estimating Occupational Exposures
and Environmental Releases (September 1994);
• EPA’s Application of Spray Polyurethane Foam Insulation – Generic Scenario for Estimating
Occupational Exposures and Environmental Releases (March 2019;
• EPA’s Industry Profile for the Flexible Polyurethane Foam Industry- Generic Scenario for
Estimating Occupational Exposures and Environmental Releases (February 2004); and,
• EPA’s Industry Profile for the Rigid Polyurethane Foam Industry – Draft Generic Scenario
for Estimating Occupational Exposures and Environmental Releases (September 2004).
OECD Emission Scenario Documents are available at the following: https://www.epa.gov/tsca-
screening-tools/chemsteer-chemical-screening-tool-exposures-and-environmental-releases
ESDs that contain information that may be related to the potential uses of TPP include, but are
not limited to:

45
• OECD’s Complementing Document to the ESD On Plastic Additives: Plastic Additives
During the Use of End Products (May 2019);
• OECD’s Complementing Document for ESD on Coating Industry: Application of Paint
Solvents for Industrial Coating (December 2015);
• OECD’s ESD on the Chemical Industry (September 2011);
• OECD’s ESD on Radiation Curable Coating, Inks, and Adhesives (July 2011);
• OECD’s ESD on Plastic Additives (July 2009); and
• OECD’s ESD on Coating Industry (Paints, Lacquers and Varnishes) (July 2009).
If ESDs and GSs are not available, other methods may be considered. EPA may also perform
supplemental targeted searches of peer-reviewed or gray literature for applicable models and
associated parameters that EPA may use to estimate releases for certain conditions of use.
Additionally, for conditions of use where no measured data on releases are available, EPA may
use a variety of methods including the application of default assumptions such as standard loss
fractions associated with drum cleaning (3%) or single process vessel cleanout (1%).
6) Map or group each condition of use to a release assessment scenario(s).
EPA has completed an initial mapping of release scenarios to relevant conditions of use as
shown in Appendix F. EPA may further refine the mapping/grouping of release scenarios based
on factors (e.g., process equipment and handling, magnitude of production volume used, and
release sources and usage rates of TPP and polymer products and formulations containing TPP,
or professional judgment) corresponding to conditions of use using reasonably available
information. EPA may perform supplemental targeted searches of peer-reviewed or gray
literature to better understand certain conditions of use to further develop release scenarios.
7) Evaluate the weight of the scientific evidence of environmental release data.
During risk evaluation, EPA plans to evaluate and integrate the exposure evidence identified in
the literature inventory using the methods described in the Application of Systematic Review in
TSCA Risk Evaluation (U.S. EPA, 2018a). EPA plans to integrate the data using systematic
review methods to assemble the relevant data, evaluate the data for quality and relevance,
including strengths and limitations, followed by synthesis and integration of the evidence.
2.7.2.2 Environmental Exposures
EPA plans to analyze the following in developing its environmental exposure assessment of TPP:
1) Review available environmental and biological monitoring data for all media relevant to
environmental exposure.
For TPP, environmental media which EPA plans to analyze are sediment, biosolids, soil, air and
water. The environmental exposure pathways which have been identified in the literature include
aquatic and terrestrial.
2) Review reasonably available information on releases to determine how modeled estimates
of concentrations near industrial point sources compare with reasonably available
monitoring data.
EPA plans to analyze and consider reasonably available environmental exposure models that
meet the scientific standards under TSCA Section 26(h) and that estimate water, sediment, and
soil concentrations alongside reasonably available water, sediment, and soil monitoring data to

46
characterize environmental exposures. Modeling approaches to estimate surface water
concentrations, sediment concentrations and soil concentrations consider the following inputs:
direct release into water, sediment, or soil, indirect release into water, sediment, or soil (i.e., air
deposition), fate and transport (partitioning within media) and characteristics of the environment
(e.g., river flow, volume of lake, meteorological data).
3) Determine applicability of existing additional contextualizing information for any
monitored data or modeled estimates during risk evaluation.
There have been changes to use patterns of TPP over the last few years. Review and characterize
monitoring data or modeled estimates to determine how representative they are of applicable use
patterns.
EPA plans to evaluate any studies which relate levels of TPP in the environment or biota with
specific sources or groups of sources.
4) Group each condition(s) of use to environmental assessment scenario(s).
EPA plans refine and finalize exposure scenarios for environmental receptors by considering
sources (use descriptors), exposure pathways including routes, and populations exposed. For
TPP, the following are noteworthy considerations in constructing exposure scenarios for
environmental receptors:
- Estimates of surface water concentrations, sediment concentrations and soil
concentrations near industrial point sources based on reasonably available monitoring
data.
- Modeling inputs such as releases into the media of interest, fate and transport and
characteristics of the environment.
- Reasonably available biomonitoring data, which could be used to compare with
species or taxa-specific toxicological benchmarks.
- Applicability of existing additional contextual information for any monitored data or
modeled estimates during risk evaluation. Review and characterize the spatial and
temporal variability, to the extent that data are reasonably available, and characterize
exposed aquatic and terrestrial populations.
- Weight of the scientific evidence of environmental occurrence data and modeled
estimates.
5) Evaluate the weight of the scientific evidence of environmental occurrence data and
modeled estimates.
During risk evaluation, EPA plans to evaluate and integrate the exposure evidence identified in
the literature inventory using methods described in the Application of Systematic Review in
TSCA Risk Evaluation (U.S. EPA, 2018a).
2.7.2.3 Occupational Exposures
EPA plans to analyze both worker and occupational non-user exposures as follows:
1) Review reasonably available exposure monitoring data for specific condition(s) of use.
EPA plans to review exposure data including workplace monitoring data collected by
government agencies such as the Occupational Safety and Health Administration (OSHA) and
the National Institute for Occupational Safety and Health (NIOSH), and monitoring data found in

47
published literature. These workplace monitoring data include personal exposure monitoring data
(direct exposures) and area monitoring data (indirect exposures).
2) Review reasonably available exposure data for surrogate chemicals that have uses,
volatility and physical and chemical properties similar to TPP.
EPA plans to review literature sources identified and if surrogate data are found, these data will
be matched to applicable conditions of use for potentially filling data gaps.
3) For conditions of use where data are limited or not reasonably available, review existing
exposure models that may be applicable in estimating exposure levels.
EPA has identified potentially relevant OECD ESDs and EPA GS corresponding to some
conditions of use. EPA plans to critically review these GS and ESDs to determine their
applicability to the conditions of use. EPA plans to perform supplemental targeted searches of
peer-reviewed or gray literature to understand those conditions of use, which may inform
identification of exposure scenarios. EPA plans to also consider the applicability of exposure
models in the Chemical Screening Tool for Exposure and Environmental Releases
(ChemSTEER) (U.S. EPA, 2013) tool that are routinely used for assessing new chemicals to
assess exposures during various conditions of use.
4) Review reasonably available data that may be used in developing, adapting or applying
exposure models to a particular risk evaluation scenario.
This will be performed after #2 and #3 are completed and based on information developed from
#2 and #3, EPA plans to evaluate relevant data to determine whether the data can be used to
develop, adapt, or apply models for specific conditions of use (and corresponding exposure
scenarios). EPA may utilize existing, peer-reviewed exposure models developed by EPA, other
government agencies, or reasonably available in the scientific literature, or EPA may elect to
develop additional models to assess specific condition(s) of use. Inhalation exposure models may
be simple box models or two-zone (near-field/far-field) models. In two-zone models, the near-
field exposure represents potential inhalation exposures to workers, and the far-field exposure
represents potential inhalation exposures to occupational non-users.
5) Consider and incorporate applicable ECs and/or PPE into exposure scenarios.
EPA plans to review potentially relevant data sources on ECs and PPE to determine their
applicability and incorporation into exposure scenarios during risk evaluation. OSHA
recommends employers utilize the hierarchy of controls to address hazardous exposures in the
workplace. The hierarchy of controls strategy outlines, in descending order of priority, the use of
elimination, substitution, engineering controls, administrative controls, and lastly personal
protective equipment (PPE). EPA plans to assess worker exposure pre- and post-implementation
of ECs, using reasonably available information on available control technologies and control
effectiveness. For example, EPA may assess worker exposure in industrial use scenarios before
and after implementation of local exhaust ventilation.
6) Map or group each condition of use to occupational exposure assessment scenario(s).
EPA has identified occupational exposure scenarios and mapped them to relevant conditions of
use (see Appendix F). As presented in Table_Apx F-1, EPA has completed an initial mapping of
exposure scenarios to condition of use. EPA plans to refine mapping or grouping of occupational
exposure scenarios based on factors (e.g., process equipment and handling, magnitude of

48
production volume used, and exposure/release sources) corresponding to conditions of use as
additional information is reviewed during risk evaluation. EPA may perform supplemental
targeted searches of peer-reviewed or gray literature to better understand certain conditions of
use to further develop exposure scenarios.
7) Evaluate the weight of the scientific evidence of occupational exposure data, which may
include qualitative and quantitative sources of information.
During risk evaluation, EPA plans to evaluate and integrate the exposure evidence identified in
the literature inventory using the methods described in the Application of Systematic Review in
TSCA Risk Evaluation (U.S. EPA, 2018a). EPA plans to rely on the weight of the scientific
evidence when evaluating and integrating occupational data. EPA plans to integrate the data
using systematic review methods to assemble the relevant data, evaluate the data for quality and
relevance, including strengths and limitations, followed by synthesis and integration of the
evidence.
2.7.2.4 Consumer Exposures
EPA plans to analyze both consumers using a consumer product and bystanders associated with the
consumer using the product as follows:
1) Group each condition of use to consumer exposure assessment scenario(s).
Refine and finalize exposure scenarios for consumers by considering combinations of sources
(ongoing consumer uses), exposure pathways including routes, and exposed populations.
For TPP, the following are noteworthy considerations in constructing consumer exposure
scenarios:
- Conditions of use
- Duration, frequency and magnitude of exposure
- Weight fraction of chemical in products
- Amount of chemical used
2) Evaluate the potential of indoor exposure pathways based on reasonably available data.
Based on physical and chemical properties of TPP and the consumer uses identified, inhalation
of particles is expected to be an important indoor exposure pathway for consumers. Other
pathways include dust ingestion and dermal contact as a result of indoor use of TPP consumer
products. Inhalation of vapor and mist and oral ingestion of liquid and mist are also possible.
EPA plans to review all reasonably available information in developing the consumer exposure
scenarios and evaluating the exposure pathways in indoor environments.
3) Review existing indoor exposure models that may be applicable in estimating indoor air
exposures.
Indoor exposure models that estimate emissions from use of consumer products are available.
These models generally consider p-chem properties (e.g., vapor pressure, molecular weight),
product specific properties (e.g., weight fraction of the chemical in the product), use patterns
(e.g., duration and frequency of use), user environment (e.g., room of use, ventilation rates), and
receptor characteristics (e.g., exposure factors, activity patterns). The OPPT’s Consumer
Exposure Model (CEM) and other similar models can be used to estimate indoor air exposures
from consumer products.

49
Models that estimate emission and migration of semi-volatile organic compounds (SVOCs) into
the indoor environment models generally consider indoor fate and transport properties such as
mass transfer as informed by the gas-phase mass transfer coefficient, the solid-phase diffusion
coefficient and the material-air partition coefficient. These properties vary based on physical and
chemical properties and properties of the material. The OPPT’s Indoor Environmental
Concentrations in Buildings with Conditioned and Unconditioned Zones (IECCU) model and
other similar models can be used to estimate indoor air and dust exposures from indoor sources.
4) Review reasonably available empirical data that may be used in developing, adapting or
applying exposure models to a particular risk evaluation scenario. For example, existing
models developed for a chemical assessment may be applicable to another chemical
assessment if model parameter data are reasonably available.
To the extent other organizations have already modeled a TPP consumer exposure scenario that
is relevant to the OPPT’s assessment, EPA plans evaluate those modeled estimates. In addition,
if other chemicals similar to TPP have been modeled for similar uses, those modeled estimates
will also be evaluated. The underlying parameters and assumptions of the models will also be
evaluated.
5) Review reasonably available consumer product-specific sources to determine how those
exposure estimates compare with each other and with indoor monitoring data reporting
TPP in specific media (e.g., indoor dust, indoor air).
The availability of TPP concentration for various conditions of use will be evaluated. This data
provides the source term for any subsequent indoor modeling. EPA plans to analyze source
attribution between overall indoor air and dust levels and various indoor sources.
6) Review reasonably available population- or subpopulation-specific exposure factors and
activity patterns to determine if potentially exposed or susceptible subpopulations need to
be further refined.
During risk evaluation, EPA plans to evaluate and integrate exposure evidence identified in the
literature inventory using the methods described in the Application of Systematic Review in
TSCA Risk Evaluation (U.S. EPA, 2018a).
7) Evaluate the weight of the scientific evidence of consumer exposure estimates based on
different approaches.
EPA plans to rely on the weight of the scientific evidence when evaluating and integrating data
related to consumer exposure. The weight of the scientific evidence may include qualitative and
quantitative sources of information. EPA plans to integrate the data using systematic review
methods to assemble the relevant data, evaluate the data for quality and relevance, including
strengths and limitations, followed by synthesis and integration of the evidence.
2.7.2.5 General Population
EPA plans to analyze general population exposures as follows:
1) Refine and finalize exposure scenarios for general population by considering sources
conditions of use, exposure pathways and routes.
For TPP, the following are noteworthy considerations in constructing exposure scenarios for the
general population:
50
- Review of reasonably available environmental and biological monitoring data for media
to which general population exposures are expected.
- For exposure pathways where data are not reasonably available, review existing exposure
modeling approaches that may be applicable in estimating exposure levels.
- Consider and incorporate applicable media-specific regulations into exposure scenarios
or modeling.
- Review reasonably available data that may be used in developing, adapting or applying
exposure models to the particular risk evaluation. For example, existing models
developed for a chemical assessment may be applicable to another chemical assessment if
model parameter data are reasonably available and relevant.
- Review reasonably available information on releases to determine how modeled
estimates of concentrations near industrial point sources compare with reasonably
available monitoring data.
- Review reasonably available population- or subpopulation-specific exposure factors and
activity patterns to determine if potentially exposed or susceptible subpopulations need
be further defined.
- Evaluate the weight of the scientific evidence of general population exposure data.
- Map or group each condition of use to general population exposure assessment
scenario(s).
EPA plans to evaluate a variety of data types to determine which types are most appropriate
when quantifying exposure scenarios. Environmental monitoring data, biomonitoring data,
modeled estimates, experimental data, epidemiological data, and survey-based data can all be
used to inform quantify exposure scenarios. EPA anticipates that there will be a range in the
potential exposures associated with the exposure scenarios identified in Section 2.6.
After refining and finalizing exposure scenarios, EPA plans quantify concentrations and/or doses
for these scenarios. The number of scenarios will depend on the conditions of use, exposure
pathways, and receptors. The number of scenarios is also dependent upon the reasonably
available data and approaches to quantify scenarios. When quantifying exposure scenarios, EPA
plans to use a tiered approach. First-tier analysis may be qualitative, semi-quantitative, or
quantitative. The results of first tier analyses inform whether scenarios require more refined
analysis. Refined analyses will be iterative and include careful consideration of variability and
uncertainty.
2) Review reasonably available environmental and biological monitoring data for exposure
pathways and media to which general population exposures are expected.
General population exposure pathways expected to be considered for TPP: ingestion of water
and food including fish and breast milk as well as dermal contact to TPP via water and inhalation
of TPP via ambient air.
3) For exposure pathways where empirical data is not reasonably available, review exposure
models that may be applicable in estimating exposure levels.
For TPP, media where exposure models will be considered for general population exposure
include models that estimate ambient air concentrations, surface water concentrations, sediment
concentrations, soil concentrations, and uptake from aquatic and terrestrial environments into
edible aquatic and terrestrial organisms.

51
4) Review reasonably available exposure modeled estimates. For example, existing models
developed for a previous TPP chemical assessment may be applicable to EPA’s assessment.
In addition, another chemical’s assessment may also be applicable if model parameter data
are reasonably available.
To the extent other organizations have already modeled TPP general population exposure
scenario that is relevant to the OPPT’s assessment, EPA plans to evaluate those modeled
estimates. In addition, if modeled estimates for other chemicals with similar physical and
chemical properties and similar uses are available, those modeled estimates will also be
evaluated. The underlying parameters and assumptions of the models will also be evaluated.
5) Review reasonably available information on releases to determine how modeled estimates
of concentrations near industrial point sources compare with reasonably available
monitoring data.
The expected releases from industrial facilities are changing over time. Any modeled
concentrations based on recent release estimates will be carefully compared with reasonably
available monitoring data to determine representativeness
6) Review reasonably available information about population- or subpopulation-specific
exposure factors and activity patterns to determine if potentially exposed or susceptible
subpopulations need to be further defined (e.g., early life and/or puberty as a potential
critical window of exposure).
For TPP, exposure scenarios that involve PESS will consider age-specific behaviors, activity
patterns, and exposure factors unique to those subpopulations. For example, children will have
different intake rates for dust, soil, and diet than adults.
7) Evaluate the weight of the scientific evidence of general population exposure estimates
based on different approaches.
During risk evaluation, EPA plans to evaluate and integrate the exposure evidence identified in
the literature inventory using the methods described in the Application of Systematic Review in
TSCA Risk Evaluation (U.S. EPA, 2018a).
Hazards (Effects)
2.7.3.1 Environmental Hazards
EPA plans to conduct an environmental hazard assessment of TPP as follows:
1) Review reasonably available environmental hazard data, including data from alternative
test methods (e.g., computational toxicology and bioinformatics; high-throughput screening
methods; data on categories and read-across; in vitro studies).
EPA plans to analyze the hazards of TPP to aquatic and terrestrial organisms, including plants,
invertebrates (e.g., insects, arachnids, mollusks, crustaceans), and vertebrates (e.g., mammals,
birds, amphibians, fish, reptiles) across exposure durations and conditions if potential
environmental hazards are identified through systematic review results and public comments.
Additional types of environmental hazard information will also be considered (e.g., analogue and
read-across data) when characterizing the potential hazards of TPP to aquatic and terrestrial
organisms.

52
EPA plans to evaluate environmental hazard data using the evaluation strategies laid out in the
Application of Systematic Review in TSCA Risk Evaluations (U.S. EPA, 2018a). The study
evaluation results will be documented in the risk evaluation phase and data from acceptable
studies will be extracted and integrated in the risk evaluation process.
Mechanistic data may include analyses of alternative test data such as novel in vitro test methods
and high throughput screening. The association between acute and chronic exposure scenarios to
the agent and each health outcome will also be integrated. Study results will be extracted and
presented in evidence tables or another appropriate format by organ/system.
2) Derive hazard thresholds for aquatic and terrestrial organisms.
Depending on the robustness of the evaluated data for a particular organism or taxa (e.g., aquatic
invertebrates), environmental hazard values (e.g., EC
x
. LC
x
, NOEC, LOEC) may be derived and
used to further understand the hazard characteristics of TPP to aquatic and terrestrial species.
Identified environmental hazard thresholds may be used to derive concentrations of concern
(COC), based on endpoints that may affect populations of organisms or taxa analyzed.
3) Evaluate the weight of the scientific evidence of environmental hazard data.
During risk evaluation, EPA plans to evaluate and integrate the environmental hazard evidence
identified in the literature inventory using the methods described in the Application of Systematic
Review in TSCA Risk Evaluations (U.S. EPA, 2018a).
4) Consider the route(s) of exposure, based on reasonably available monitoring and modeling
data and other available approaches to integrate exposure and hazard assessments.
EPA plans to consider aquatic (e.g., water and sediment exposures) and terrestrial pathways in
the TPP conceptual model. These organisms may be exposed to TPP via a number of
environmental pathways (e.g., surface water, sediment, soil, diet).
5) Consider a persistent, bioaccumulative, and toxic (PBT) assessment of TPP.
EPA plans to consider the persistence, bioaccumulation, and toxic (PBT) potential of TPP after
reviewing relevant physical and chemical properties and exposure pathways. EPA plans to assess
the reasonably available studies collected from the systematic review process relating to
bioaccumulation and bioconcentration (e.g., BAF, BCF) of TPP. In addition, EPA plans integrate
traditional environmental hazard endpoint values (e.g., LC
50
, LOEC) and exposure
concentrations (e.g., surface water concentrations, tissue concentrations) for TPP with the fate
parameters (e.g., BAF, BCF, BMF, TMF).
6) Conduct an environmental risk estimation and characterization of TPP.
EPA plans to conduct a risk estimation and characterization of TPP to identify if there are risks
to the aquatic and terrestrial environments from the measured and/or predicted concentrations of
TPP in environmental media (e.g., water, sediment, soil). Risk quotients (RQs) may be derived
by the application of hazard and exposure benchmarks to characterize environmental risk (U.S.
EPA, 1998) (U.S. EPA, 1998; Barnthouse et al., 1982). Analysis of risk for characterization
includes a confidence statement in risk estimation which qualitative judgment describing the
certainty of the risk estimate considering the strength the evidence scores for hazard and
exposure and the limitations, and relevance.

53
2.7.3.2 Human Health Hazards
EPA plans to analyze human health hazards as follows:
1) Review reasonably available human health hazard data, including data from alternative
test methods (e.g., computational toxicology and bioinformatics; high-throughput screening
methods; data on categories and read-across; in vitro studies; systems biology).
EPA plans to evaluate human health studies using the evaluation strategies laid out in the
Application of Systematic Review in TSCA Risk Evaluations (U.S. EPA, 2018a) and updates to
the epidemiological data quality criteria released with the first ten risk evaluations. The study
evaluation results will be documented in the risk evaluation phase and data from acceptable
studies will be extracted and integrated in the risk evaluation process.
Mechanistic data may include analyses of alternative test data such as novel in vitro test methods
and high throughput screening. The association between acute and chronic exposure scenarios to
the agent and each health outcome will also be integrated. Study results will be extracted and
presented in evidence tables or another appropriate format by organ/system.
2) In evaluating reasonably available data, determine whether particular human receptor
groups may have greater susceptibility to the chemical’s hazard(s) than the general
population.
Reasonably available human health hazard data will be evaluated to ascertain whether some
human receptor groups may have greater susceptibility than the general population to TPP
hazard(s). Susceptibility of particular human receptor groups to TPP will be determined by
evaluating information on factors that influence susceptibility.
EPA has reviewed some sources containing hazard information associated with susceptible
populations, and lifestages such as pregnant women and infants. Pregnancy (i.e., gestation) and
childhood are potential susceptible lifestages for TPP exposure. EPA may quantify these
differences in the risk evaluation following further evaluation of the reasonably available data
and information.
3) Conduct hazard identification (the qualitative process of identifying non-cancer and cancer
endpoints) and dose-response assessment (the quantitative relationship between hazard
and exposure) for identified human health hazard endpoints.
Human health hazards from acute and chronic exposures will be identified by evaluating the
human and animal data that meet the systematic review data quality criteria described in the
Application of Systematic Review in TSCA Risk Evaluations (U.S. EPA, 2018a). Hazards
identified by studies meeting data quality criteria will be grouped by routes of exposure relevant
to humans (e.g., oral, dermal, inhalation) and by the cancer and noncancer endpoints identified in
Section 2.4.2.

54
Dose-response assessment will be performed in accordance with EPA guidance (U.S. EPA,
2012a, 2011a, 1994) developing points of departure (POD) for either margins of exposure
(MOEs), cancer slope factors (CSFs), oral slope factors (OSFs), and/or inhalation unit risks
(IURs). Dose-response analyses may be used if the data meet data quality criteria and if
additional information on the identified hazard endpoints are not reasonably available or would
not alter the analysis
The cancer mode of action (MOA) analyses determine the relevancy of animal data to human
risk and how data can be quantitatively evaluated. If cancer hazard is determined to be applicable
to TPP, EPA plans to evaluate information on genotoxicity and the MOA for all cancer endpoints
to determine the appropriate approach for quantitative cancer assessment in accordance with the
U.S. EPA Guidelines for Carcinogen Risk Assessment (U.S. EPA, 2005a). In accordance with
EPA’s Supplemental Guidance for Assessing Susceptibility from Early-life Exposures to
Carcinogens (U.S. EPA, 2005b), EPA plans to determine whether age-dependent adjustment
factors (ADAFs) are appropriate for TPP for specific conditions of use based upon potential
exposures to children.
4) Derive points of departure (PODs) where appropriate; conduct benchmark dose modeling
depending on the reasonably available data. Adjust the PODs as appropriate to conform
(e.g., adjust for duration of exposure) to the specific exposure scenarios evaluated.
Hazard data will be evaluated to determine the type of dose-response modeling that is applicable.
Where modeling is feasible, a set of dose-response models that are consistent with a variety of
potentially underlying biological processes will be applied to empirically model the dose-
response relationships in the range of the observed data consistent with EPA’s Benchmark Dose
Technical Guidance Document (U.S. EPA, 2012a). Where dose-response modeling is not
feasible, NOAELs or LOAELs will be identified. Non-quantitative data will also be evaluated
for contribution to weight of the scientific evidence or for evaluation of qualitative endpoints that
are not appropriate for dose-response assessment.
EPA plans to evaluate whether the reasonably available PBPK and empirical kinetic models are
adequate for route-to-route and interspecies extrapolation of the POD, or for extrapolation of the
POD to standard exposure durations (e.g., lifetime continuous exposure). If application of the
PBPK model is not possible, oral PODs may be adjusted by BW
3/4
scaling in accordance with
U.S. EPA (2011b), and inhalation PODs may be adjusted by exposure duration and chemical
properties in accordance with U.S. EPA (1994).
5) Evaluate the weight of the scientific evidence of human health hazard data.
During risk evaluation, EPA plans to evaluate and integrate the human health hazard evidence
identified in the literature inventory under acute and chronic exposure conditions using the
methods described in the Application of Systematic Review in TSCA Risk Evaluations (U.S. EPA,
2018a).
6) Consider the route(s) of exposure (e.g., oral, inhalation, dermal), reasonably available
route-to-route extrapolation approaches; biomonitoring data; and approaches to correlate
internal and external exposures to integrate exposure and hazard assessment.

55
At this stage of review, EPA believes there will be sufficient reasonably available data to
conduct a dose-response analysis and/or benchmark dose modeling for the oral route of
exposure. EPA plans to also evaluate any potential human health hazards following dermal and
inhalation exposure to TPP, which could be important for worker, consumer and general
population risk analysis. Reasonably available data will be assessed to determine whether or not
a point of departure can be identified for the dermal and inhalation routes.
If sufficient reasonably available toxicity studies are not identified through the systematic review
process to assess risks from inhalation or dermal exposure, then a route-to-route extrapolation
may be needed. The preferred approach is to use a PBPK model (U.S. EPA, 2006a). Without an
adequate PBPK model, considerations regarding the adequacy of data for route-to-route
extrapolation are described in Methods for Derivation of Inhalation Reference Concentrations
and Application of Inhalation Dosimetry (U.S. EPA, 1994). EPA may use these considerations
when determining whether to extrapolate from the oral to the inhalation route of exposure.
Similar approaches for oral-to-dermal route extrapolation are described in EPA guidance
document Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation
Manual (Part E, Supplemental Guidance for Dermal Risk Assessment) (U.S. EPA, 2004).
If there are acceptable inhalation data after completion of systematic review, EPA may also
consider extrapolating from the inhalation to the dermal route if first-pass metabolism through
the liver via the oral route is expected because in that case, use of data from the oral route is not
recommended (U.S. EPA, 1994). EPA may also consider inhalation-to-dermal route
extrapolation if an inhalation toxicity study with a sensitive hazard endpoint is used to evaluate
risks. Based on these considerations, EPA extrapolated from the inhalation to the dermal route
for several of the first ten risk evaluations under amended TSCA, including methylene chloride
(U.S. EPA, 2020d) and carbon tetrachloride (U.S. EPA, 2020b).
7) Conduct a human health risk estimation and characterization of TPP.
Analysis of risk for characterization includes a confidence statement in risk estimation. This
confidence statement is based on qualitative judgment describing the certainty of the risk
estimate considering the strength of the evidence scores for hazard and exposure along with their
limitations and relevance. The lowest confidence evaluation for either hazard or exposure will
drive the overall confidence estimate.
Summary of Risk Approaches for Characterization
Risk characterization is an integral component of the risk assessment process for both environmental and
human health risks. EPA plans derive the risk characterization in accordance with EPA’s Risk
Characterization Handbook (U.S. EPA, 2000a). As defined in EPA’s Risk Characterization Policy, “the
risk characterization integrates information from the preceding components of the risk evaluation and
synthesizes an overall conclusion about risk that is complete, informative and useful for decision
makers” (U.S. EPA, 2000a). Risk characterization is considered to be a conscious and deliberate process
to bring all important considerations about risk, not only the likelihood of the risk but also the strengths
and limitations of the assessment, and a description of how others have assessed the risk into an
integrated picture.
The level of information contained in each risk characterization varies according to the type of
assessment for which the characterization is written. Regardless of the level of complexity or
information, the risk characterization for TSCA risk evaluations will be prepared in a manner that is

56
transparent, clear, consistent, and reasonable (U.S. EPA, 2000b), and consistent with the requirements of
the Procedures for Chemical Risk Evaluation Under the Amended Toxic Substances Control Act (82 FR
33726, July 20, 2017). As discussed in 40 CFR 702.43, risk characterization has a number of
considerations. This is the step where EPA integrates the hazard and exposure assessments into risk
estimates for the identified populations (including any PESS) and ecological characteristics and weighs
the scientific evidence for the identified hazards and exposures. The risk characterization does not
consider costs or other nonrisk factors, and takes into account, “where relevant, the likely duration,
intensity, frequency, and number of exposures under the condition(s) of use ….” The risk
characterization also summarizes the following considerations: (1) uncertainty and variability in each
step of the risk evaluation; (2) data quality, and any applicable assumptions used; (3) alternative
interpretations of data and analyses, where appropriate; and (4) any considerations for environmental
risk evaluations, if necessary (e.g., related to nature and magnitude of effects).
EPA plans to also be guided by EPA’s Information Quality Guidelines (U.S. EPA, 2002) as it provides
guidance for presenting risk information. Consistent with those guidelines, in the risk characterization,
EPA plans to also identify: (1) each population addressed by an estimate of applicable risk effects; (2)
the expected risk or central estimate of risk for the PESS affected; (3) each appropriate upper-bound or
lower bound estimate of risk; (4) each significant uncertainty identified in the process of the assessment
of risk effects and the studies that would assist in resolving the uncertainty; and (5) peer reviewed
studies known to the Agency that support, are directly relevant to, or fail to support any estimate of risk
effects and the methodology used to reconcile inconsistencies in the scientific information.
2.8 Peer Review
Peer review will be conducted in accordance with EPA's regulatory procedures for chemical risk
evaluations, including using EPA’s Peer Review Handbook (U.S. EPA, 2015a) and other methods
consistent with Section 26 of TSCA (see 40 CFR 702.45). As explained in the Risk Evaluation Rule, the
purpose of peer review is for the independent review of the science underlying the risk assessment. Peer
review will therefore address aspects of the underlying science as outlined in the charge to the peer
review panel such as hazard assessment, assessment of dose-response, exposure assessment, and risk
characterization. The draft risk evaluation for TPP will be peer reviewed.

57
REFERENCES
Anderson, C; Wischer, D; Schmieder, A; Spiteller, M. (1993). Fate of triphenyl phosphate in soil.
Chemosphere 27: 869-879.
ATSDR (Agency for Toxic Substances and Disease Registry). (2017). Toxicological profile for1-
bromopropane. Atlanta, GA: Division of Toxicology and Human Health Sciences,
Environmental Toxicology Branch. https://www.atsdr.cdc.gov/ToxProfiles/tp209.pdf
Barnthouse, LW; DeAngelis, DL; Gardner, RH; O'Neill, RV; Suter, GW; Vaughan, DS. (1982).
Methodology for environmental risk analysis. (ORNL/TM-8167). Oak Ridge, TN: Oak Ridge
National Laboratory.
Central Garden & Pet. (2017). Safety data sheet: Zodiac Flea & Tick Collar for Dogs & Puppies.
Cherrie, JW; Semple, S; Christopher, Y; Saleem, A; Hughson, GW; Philips, A. (2006). How important
is inadvertent ingestion of hazardous substances at work? Ann Occup Hyg 50: 693-704.
http://dx.doi.org/10.1093/annhyg/mel035
EWG (Environmental Working Group). (2019). Skin Deep Cosmetics Database: Triphenyl phosphate. .
https://www.ewg.org/skindeep/ingredient/706709/TRIPHENYL_PHOSPHATE/
Howard, BE; Phillips, J; Miller, K; Tandon, A; Mav, D; Shah, MR; Holmgren, S; Pelch, KE; Walker, V;
Rooney, AA; Macleod, M; Shah, RR; Thayer, K. (2016). SWIFT-Review: a text-mining
workbench for systematic review. Syst Rev 5: 87. http://dx.doi.org/10.1186/s13643-016-0263-z
Howard, P; Deo, P. (1979). Degradation of aryl phosphates in aquatic environments. Bull Environ
Contam Toxicol 3: 337-344.
HSDB (Hazardous Substances Data Bank). (2019). Triphenyl phosphate, CASRN: 115-86-6. U.S.
Department of Health and Human Services, National Institutes of Health, National Library of
Medicine. https://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+2536
Lombardo, P; Egry, IJ. (1979). Identification and gas-liquid chromatographic determination of aryl
phosphate residues in environmental samples. J Assoc Offic Anal Chem 62: 47-51.
Mayer, FL; Adams, WJ; Finley, MT; Michael, PR; Mehrle, PM; Saeger, VW. (1981). Phosphate ester
hydraulic fluids: an aquatic environmental assessment of Pydrauls 50E and 115E. In DR
Branson; KL Dickson (Eds.), Aquatic Toxicology and Hazard Assessment: Fourth conference
(pp. 103-123). Philadelphia, PA: American Society for Testing and Materials.
https://search.proquest.com/docview/13835125?accountid=171501
Muir, DCG; Yarechewski, AL; Grift, NP. (1983). Environmental dynamics of phosphate esters. III.
Comparison of the bioconcentration of four triaryl phosphates by fish. Chemosphere 12: 155-
166. http://dx.doi.org/10.1016/0045-6535(83)90159-5
NIOSH. (2016). Updated Immediately Dangerous To Life or Health (IDLH) Values (as of 2016).
Atlanta, GA. http://www.cdc.gov/niosh/idlh/intridl4.html
NIOSH. (2019). NIOSH pocket guide to chemical hazards. Index of chemical abstracts service registry
numbers (CAS No.). Atlanta, GA: Center for Disease Control and Prevention, U.S. Department
of Health, Education and Welfare. http://www.cdc.gov/niosh/npg/npgdcas.html
NITE (National Institute of Technology and Evaluation). (2019). Chemical Risk Information Platform
(CHRIP): CASRN 115-86-6. Japan.
http://www.safe.nite.go.jp/english/sougou/view/ComprehensiveInfoDisplay_en.faces
NLM. (2018). PubChem: Hazardous Substance Data Bank: Triphenyl phosphate, 115-86-6 [Website].
https://pubchem.ncbi.nlm.nih.gov/compound/8289#source=HSDB
OECD (Organisation for Economic Co-operation and Development). (2002). SIDS Initial Assessment
Report: Triphenyl phosphate (pp. 151). Paris, France.
https://hpvchemicals.oecd.org/ui/handler.axd?id=E23395DC-ED57-4822-B9C4-7178045C3C97

58
OECD (Organisation for Economic Co-operation and Development). (2009). Emission scenario
documents on coating industry (paints, lacquers and varnishes). (JT03267833). Paris, France.
OSHA (Occupational Safety and Health Administration). (2019). Permissible exposure limits: OSHA
annotated table Z-1. United States Department of Labor, Occupational Safety & Health
Administration. https://www.osha.gov/dsg/annotated-pels/tablez-1.html
RSC. (2019). ChemSpider: Triphenyl phthalate [Website]. http://www.chemspider.com/Chemical-
Structure.7988.html?rid=9b344cd2-9db5-4a46-8ff9-b01ddff46010
Rumble J. R., E. (2018). Triphenyl phosphate. In CRC Handbook of Chemistry and Physics (99 ed.).
Boca Raton, FL: CRC Press. Taylor & Francis Group.
Saeger, VH; Kaley. (1979). Environmental fate of selected phosphate esters. Environ Sci Technol 13:
840-844.
Snyder, R. (1990). Nitrogen and Phosphorus Solvents. In Ethel Browning's Toxicity and Metabolism of
Industrial Solvents (2nd ed.). Amsterdam-New York-Oxford: Elsevier.
Stapleton, HM; Klosterhaus, S; Eagle, S; Fuh, J; Meeker, JD; Blum, A; Webster, TF. (2009). Detection
of organophosphate flame retardants in furniture foam and U.S. house dust. Environ Sci Technol
43: 7490-7495. http://dx.doi.org/10.1021/es9014019
Stapleton, HM; Klosterhaus, S; Keller, A; Ferguson, P; van Bergen, S; Cooper, E; Webster, TF; Blum,
A. (2011). Identification of Flame Retardants in Polyurethane Foam Collected from Baby
Products. Environ Sci Technol 45: 5323-5331. http://dx.doi.org/10.1021/es2007462
U.S. EPA (U.S. Environmental Protection Agency). (1994). Methods for derivation of inhalation
reference concentrations and application of inhalation dosimetry [EPA Report]. (EPA/600/8-
90/066F). Research Triangle Park, NC: U.S. Environmental Protection Agency, Office of
Research and Development, Office of Health and Environmental Assessment, Environmental
Criteria and Assessment Office.
https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=71993&CFID=51174829&CFTOKEN=2
5006317
U.S. EPA (U.S. Environmental Protection Agency). (1998). Guidelines for ecological risk assessment
[EPA Report]. (EPA/630/R-95/002F). Washington, DC: U.S. Environmental Protection Agency,
Risk Assessment Forum.
U.S. EPA (U.S. Environmental Protection Agency). (2000a). Science policy council handbook: Risk
characterization (pp. 1-189). (EPA/100/B-00/002). Washington, D.C.: U.S. Environmental
Protection Agency, Science Policy Council. https://www.epa.gov/risk/risk-characterization-
handbook
U.S. EPA (U.S. Environmental Protection Agency). (2000b). Science Policy Council handbook: Risk
characterization handbook [EPA Report]. (EPA/100/B-00/002). Washington, D.C.: U.S.
Environmental Protection Agency, Science Policy Council. https://www.epa.gov/risk/risk-
characterization-handbook
U.S. EPA (U.S. Environmental Protection Agency). (2002). Guidelines for ensuring and maximizing the
quality, objectivity, utility, and integrity of information disseminated by the Environmental
Protection Agency. (EPA/260/R-02/008). Washington, DC: U.S. Environmental Protection
Agency, Office of Environmental Information. https://www.epa.gov/sites/production/files/2017-
03/documents/epa-info-quality-guidelines.pdf
U.S. EPA (U.S. Environmental Protection Agency). (2004). Risk Assessment Guidance for Superfund
(RAGS), Volume I: Human health evaluation manual, (Part E: Supplemental guidance for
dermal risk assessment): Final. (EPA/540/R/99/005). Washington, DC.
http://www.epa.gov/oswer/riskassessment/ragse/index.htm

59
U.S. EPA (U.S. Environmental Protection Agency). (2005a). Guidelines for carcinogen risk assessment
[EPA Report]. (EPA/630/P-03/001B). Washington, DC: U.S. Environmental Protection Agency,
Risk Assessment Forum. https://www.epa.gov/sites/production/files/2013-
09/documents/cancer_guidelines_final_3-25-05.pdf
U.S. EPA (U.S. Environmental Protection Agency). (2005b). Supplemental guidance for assessing
susceptibility from early-life exposure to carcinogens [EPA Report]. (EPA/630/R-03/003F).
Washington, DC: U.S. Environmental Protection Agency, Risk Assessment Forum.
https://www3.epa.gov/airtoxics/childrens_supplement_final.pdf
U.S. EPA. (U.S. Environmental Protection Agency). (2006a). Approaches for the application of
physiologically based pharmacokinetic (PBPK) models and supporting data in risk assessment
(Final Report) [EPA Report] (pp. 1-123). (EPA/600/R-05/043F). Washington, DC: U.S.
Environmental Protection Agency, Office of Research and Development, National Center for
Environmental Assessment. http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=157668
U.S. EPA (U.S. Environmental Protection Agency). (2006b). A framework for assessing health risk of
environmental exposures to children (pp. 1-145). (EPA/600/R-05/093F). Washington, DC: U.S.
Environmental Protection Agency, Office of Research and Development, National Center for
Environmental Assessment. http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=158363
U.S. EPA (U.S. Environmental Protection Agency). (2011a). Exposure factors handbook: 2011 edition
[EPA Report]. (EPA/600/R-090/052F). Washington, DC.
http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=236252
U.S. EPA. (U.S. Environmental Protection Agency). (2011b). Recommended use of body weight 3/4 as
the default method in derivation of the oral reference dose (pp. 1-50). (EPA/100/R-11/0001).
Washington, DC: U.S. Environmental Protection Agency, Risk Assessment Forum, Office of the
Science Advisor. https://www.epa.gov/sites/production/files/2013-09/documents/recommended-
use-of-bw34.pdf
U.S. EPA (U.S. Environmental Protection Agency). (2012a). Benchmark dose technical guidance.
(EPA/100/R-12/001). Washington, DC: U.S. Environmental Protection Agency, Risk
Assessment Forum. https://www.epa.gov/risk/benchmark-dose-technical-guidance
U.S. EPA (U.S. Environmental Protection Agency). (2012b). Estimation Programs Interface Suite for
Microsoft Windows, v 4.11 [Computer Program]. Washington, DC. https://www.epa.gov/tsca-
screening-tools/epi-suitetm-estimation-program-interface
U.S. EPA (U.S. Environmental Protection Agency). (2013). ChemSTEER user guide - Chemical
screening tool for exposures and environmental releases. Washington, D.C.
https://www.epa.gov/sites/production/files/2015-05/documents/user_guide.pdf
U.S. EPA (U.S. Environmental Protection Agency). (2014a). Flame retardant alternatives for
hexabromocyclododecane (HBCD) [EPA Report]. (EPA/740/R-14/001). Washington, D.C.
http://www2.epa.gov/saferchoice/partnership-evaluate-flame-retardant-alternatives-hbcd-
publications
U.S. EPA (U.S. Environmental Protection Agency). (2014b). Use of additives in the thermoplastic
converting industry-Generic scenario for estimating occupational exposures and environmental
releases (Draft).
U.S. EPA (U.S. Environmental Protection Agency). (2015a). Peer review handbook [EPA Report] (4th
ed.). (EPA/100/B-15/001). Washington, DC: U.S. Environmental Protection Agency, Science
Policy Council. https://www.epa.gov/osa/peer-review-handbook-4th-edition-2015
U.S. EPA (U.S. Environmental Protection Agency). (2015b). Science Policy Council peer review
handbook [EPA Report] (4th ed.). (EPA/100/B-15/001). Washington, DC: U.S. Environmental
Protection Agency, Science Policy Council.
60
U.S. EPA (U.S. Environmental Protection Agency). (2017). Procedures for chemical risk evaluation
under the amended Toxic Substances Control Act. Final Rule Federal Registrar 82: 33726-
33753. Fed Reg 82.
U.S. EPA (U.S. Environmental Protection Agency). (2018a). Application of systematic review in TSCA
risk evaluations. (740-P1-8001). Washington, DC: U.S. Environmental Protection Agency,
Office of Chemical Safety and Pollution Prevention.
https://www.epa.gov/sites/production/files/2018-
06/documents/final_application_of_sr_in_tsca_05-31-18.pdf
U.S. EPA (U.S. Environmental Protection Agency). (2018b). Problem formulation of the risk evaluation
for 1-bromopropane. (EPA-740-R1-7019). Washington, DC: Office of Chemical Safety and
Pollution Prevention, United States Environmental Protection Agency.
https://www.epa.gov/sites/production/files/2018-06/documents/1bp_problem_formulation_05-
31-18.pdf
U.S. EPA (U.S. Environmental Protection Agency). (2018c). Problem formulation of the risk evaluation
for cyclic aliphatic bromides cluster (HBCD). (EPA-740-R1-7012). Washington, DC: Office of
Chemical Safety and Pollution Prevention, United States Environmental Protection Agency.
https://www.epa.gov/sites/production/files/2018-06/documents/hbcd_problem_formulation_05-
31-18.pdf
U.S. EPA (U.S. Environmental Protection Agency). (2018d). Problem formulation of the risk evaluation
for methylene chloride (dichloromethane, DCM). (EPA-740-R1-7016). Washington, DC: Office
of Chemical Safety and Pollution Prevention, United States Environmental Protection Agency.
https://www.epa.gov/sites/production/files/2018-06/documents/mecl_problem_formulation_05-
31-18.pdf
U.S. EPA (U.S. Environmental Protection Agency). (2019a). Chemical Data Reporting (2012 and 2016
CBI CDR database). Washington, DC.: Office of Pollution Prevention and Toxics.
U.S. EPA (U.S. Environmental Protection Agency). (2019b). Chemical Data Reporting (2012 and 2016
CBI CDR database).
U.S. EPA (U.S. Environmental Protection Agency). (2019c). Chemistry Dashboard Information for
Triphenyl phosphate. 115-86-6 [Website].
https://comptox.epa.gov/dashboard/dsstoxdb/results?search=DTXSID1021952
U.S. EPA (U.S. Environmental Protection Agency). (2019d). Proposed designation of triphenyl
phosphate (CASRN 115-86-6) as a high-priority substance for risk evaluation. U.S.
Environmental Protection Agency, Office of Chemical Safety and Pollution Prevention.
https://www.epa.gov/sites/production/files/2019-08/documents/triphenylphosphate_115-86-
6_high-priority_proposeddesignation_082319_0.pdf
U.S. EPA (U.S. Environmental Protection Agency). (2020a). Chemical data reporting (2012 and 2016
Public CDR database) [Database]. Washington, DC: U.S. Environmental Protection Agency,
Office of Pollution Prevention and Toxics. ChemView: July 2020.
https://chemview.epa.gov/chemview
U.S. EPA. (U.S. Environmental Protection Agency). (2020b). Draft risk evaluation for carbon
tetrachloride (methane, tetrachloro-); CASRN: 56-23-5 (pp. 1-301). (EPA-740-R1-8014). Office
of Chemical Safety and Pollution Prevention, U.S. Environmental Protection Agency.
https://nepis.epa.gov/Exe/ZyPDF.cgi/P100YHUW.PDF?Dockey=P100YHUW.PDF
U.S. EPA. (U.S. Environmental Protection Agency). (2020c). Draft Scope of the risk evaluation for
Triphenyl Phosphate CASRN 115-86-6 [EPA Report]. (EPA-740-D-20-010). Washington, DC.
https://www.epa.gov/sites/production/files/2020-04/documents/casrn-115-86-
6_phosphoric_acid_triphenyl_ester_tpp_draft_scope.pdf

61
U.S. EPA. (U.S. Environmental Protection Agency). (2020d). Risk evaluation for methylene chloride
(dichloromethane, dcm); CASRN: 75-09-2 (pp. 1-753). (EPA-740-R1-8010). Office of Chemical
Safety and Pollution Prevention, U.S. Environmental Protection Agency.
https://www.epa.gov/sites/production/files/2020-
06/documents/1_mecl_risk_evaluation_final.pdf
UK Environment Agency. (2009). Environmental risk evaluation report: Triphenyl phosphate (CAS no.
115-86-6). Bristol, UK: The Environment Agency.
https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file
/290862/scho0809bquk-e-e.pdf
USGS (U.S. Geological Survey). (1991a). USGS Monitoring Data: National Water Quality Monitoring
Council [Database]. http://www.waterqualitydata.us/portal/
USGS (U.S. Geological Survey). (1991b). USGS Monitoring Data: National Water Quality Monitoring
Council - Air.
https://www.waterqualitydata.us/portal/#siteType=Aggregate%20groundwater%20use&sample
Media=Water&mimeType=csv&dataProfile=activityAll
USGS (U.S. Geological Survey). (1991c). USGS Monitoring Data: National Water Quality Monitoring
Council - Groundwater.
https://www.waterqualitydata.us/portal/#siteType=Aggregate%20groundwater%20use&sample
Media=Water&mimeType=csv&dataProfile=activityAll
USGS (U.S. Geological Survey). (1991d). USGS Monitoring Data: National Water Quality Monitoring
Council - Sediment.
https://www.waterqualitydata.us/portal/#sampleMedia=Sediment&mimeType=csv
USGS (U.S. Geological Survey). (1991e). USGS Monitoring Data: National Water Quality Monitoring
Council - Soil. https://www.waterqualitydata.us/portal/#sampleMedia=Soil&mimeType=csv
USGS (U.S. Geological Survey). (1991f). USGS Monitoring Data: National Water Quality Monitoring
Council - Surface Water.
https://www.waterqualitydata.us/portal/#siteType=Aggregate%20surface-water-
use&sampleMedia=Water&mimeType=csv
USGS (U.S. Geological Survey). (1991g). USGS Monitoring Data: National Water Quality Monitoring
Council - Tissue.
https://www.waterqualitydata.us/portal/#sampleMedia=Tissue&mimeType=csv
Weil, ED. (2001). Kirk-Othmer Encyclopedia of Chemical Technology. Flame Retardants, Phosphorus.
New York, NY: John Wiley & Sons.
http://dx.doi.org/https://onlinelibrary.wiley.com/doi/abs/10.1002/0471238961.160815192305091
2.a01.pub2
62
APPENDICES
ABBREVIATED METHODS FOR SEARCHING AND
SCREENING
Literature Search of Publicly Available Databases
A.1.1 Search Term Genesis and Chemical Verification
To develop the chemical terms for the subsequent literature search for TPP, several online sources were
queried.
• California Department of Pesticide Regulation:
https://www.cdpr.ca.gov/docs/chemical/monster2.htm
• USEPA Chemistry Dashboard: https://comptox.epa.gov/dashboard
• University of Hertfordshire PPDB: Pesticide Properties DataBase:
https://sitem.herts.ac.uk/aeru/ppdb/en/search.htm
• USEPA Reregistration Eligibility Decision (RED) documents:
https://archive.epa.gov/pesticides/reregistration/web/html/status.html
• Office of Pesticide Programs Pesticide Chemical Search:
https://ofmpub.epa.gov/apex/pesticides/f?p=CHEMICALSEARCH:1
• Food and Agriculture Organization of the United Nations: http://www.fao.org/home/en/
• PAN Pesticides Database: http://www.pesticideinfo.org/Search_Chemicals.jsp
Prior to inclusion in the search term string, all forms of chemical names were subjected to verification
from several potential sources (e.g., US EPA Chemistry Dashboard, STN International-CAS; see
complete list of sources for chemical verification in Table_Apx A-1. From these sources, all chemical
names, synonyms, CAS number(s), trade names, etc. were documented and used to generate terms for
database searches.
Table_Apx A-1. Sources of Verification for Chemical Names and Structures
CHEMICAL SOURCE
CONTENTS
DOCUMENT
LOCATION
Chemistry Dashboard
(https://comptox.epa.gov/dashboard)
CAS Numbers, Synonyms, Structures, Properties,
Environmental Fate and Transport.
Online
Dictionary of Chemical Names and Synonyms
Wide assortment of chemical compounds by chemical
name and synonym, has CAS index and some
structure data
ECOTOX
Farm Chemicals Handbook-1992
Pesticide information, CAS numbers and synonyms,
some structure data
***Sometimes CAS number presented for a
compound is for the main constituent only
ECOTOX
OPPT SMILES Verification Source
Structure Data
Electronic
verification
RTECS (Registry of Toxic Effects of
chemical substance, 1983-84 ed., 2 vols)
Chemical names, synonyms and CAS numbers
ECOTOX
63
CHEMICAL SOURCE
CONTENTS
DOCUMENT
LOCATION
Sigma – Aldrich website58784
http://www.sigma-aldrich.com
Organic and inorganic Compounds by chemical name,
has CAS index and some structure and Physical
Property data
Online
STN International (CAS) 1994
***Most complete source of chemical name, synonym
and structure information, no physical properties
Online
The Pesticide Manual 10th edition, 1994
Pesticide Compounds by chemical name, synonym,
product code, has CAS index and some structure and
Physical Property data
ECOTOX
TSCA (Toxic Substances Control Act
Chemical Substance Inventory, 1985 ed., 5
vols)
Chemical names, synonyms and CAS numbers
ECOTOX
World Wide Web (misc. web sources) A copy
of the verification page is saved to the
Attachments tab of the chemical entry. This
includes company MSDS sheets or Chemical
Labels.
Chemical names, synonyms and CAS numbers
Online
California Department of Pesticide Regulation
(http://www.cdpr.ca.gov/dprdatabase.htm)
Multiple databases containing chemicals, pesticides,
companies, products, etc.
Online
PAN Pesticide Database
(http://www.pesticideinfo.org/Search_Chemic
als.jsp )
Pesticides searchable by name or CAS #. Includes
CAS #, Name, synonyms, targets, toxicity data,
related chemicals and regulatory information.
Online
US EPA Office of Pesticide Programs
Pesticide Fate Database – No web access
available. An electronic copy of the data file is
located at the Contractor site:
PFATE_37_Tables.mdb.
Multiple databases containing chemicals, pesticides,
companies, products, etc.
Online
A.1.2 Publicly Available Database Searches
The databases listed below were searched for literature containing the chemical search terms. Database
searching occurred during April and May of 2019 by an information specialist and the results were
stored in the Health and Environmental Research Online (HERO) database and assigned a HERO
reference identification number.
6
The present literature search focused only on the chemical name
(including synonyms and trade names) with no additional limits. Full details of the search strategy for
each database are presented in Appendix A.1.2.1.
After initial deduplication in HERO
7
, these studies were imported into SWIFT Review software
(Howard et al., 2016) to identify those references most likely to be applicable to each discipline area (i.e.
consumer, environmental, and general population exposure, occupational exposure and environmental
releases, environmental hazards, human health hazards, and fate and physical chemistry).
A.1.2.1 Query Strings for the Publicly Available Database Searches on TPP
Table_Apx A-2 presents a list of the data sources, the search dates and number of peer-reviewed
references resulting from the searches for TPP. The sources are found as online databases and the
resulting references were gathered and uploaded into the EPA Health and Environmental Research
6
EPA’s HERO database provides access to the scientific literature behind EPA science assessments. The database includes
more than 600,000 scientific references and data from the peer-reviewed literature used by EPA to develop its regulations.
7
Deduplication in HERO involves first determining whether a matching unique ID exists (e.g., PMID, WOSid, or DOI). If
one matches one that already exists in HERO, HERO will tag the existing reference instead of adding the reference again.
Second, HERO checks if the same journal, volume, issue and page number are already in HERO. Third, HERO matches on
the title, year, and first author. Title comparisons ignore punctuation and case.
64
Online (HERO) database for literature screening.
Table_Apx A-2. Summary of Data Sources, Search Dates and Number of Peer-Reviewed
Literature Search Results for TPP
Source
Date of Search
Number of References
Current Contents
05/02/2019
497
ProQuest CSA
05/02/2019
1092
Dissertation Abstracts
05/03/2019
6
Science Direct
05/02/2019
258
Agricola
05/02/2019
270
TOXNET
05/02/2019
624
UNIFY
05/03/2019
162
Totals:
2909
GENERAL:
General search terms were compiled and used in the search strategies for each of the databases/sources
listed below. Based upon the online search manuals for the respective databases/sources, it was
necessary to construct searches as noted for each of the sources. The search terms are listed below in full
for each source and noted if the general search terms or other search terms were used.
"Antioxidant TTP" OR "BRN 1888236" OR "Celluflex TPP" OR "DHPF 005" OR "Disflamoll TP" OR
"NSC 57868" OR "O,O,O-Triphenyl phosphate" OR "Phenyl phosphate" OR "Phoscon FR 903N" OR
"Phosflex TPP" OR "Phosphoric acid, triphenyl ester" OR "Phosphoric acid, triphenyl ester radical
ion(1+)" OR "Reofos TPP" OR "Sumilizer TPP" OR "Triphenol phosphate" OR "Triphenoxyphosphine
oxide" OR "Triphenyl phosphate" OR "Triphenylphosphat" OR "Triphenylphosphate" OR "UN 3077"
OR "UNII-YZE19Z66EA" OR "Wako TPP" OR "WSFR-TPP"
CURRENT CONTENTS CONNECT: (access.webofknowledge.com)
General Search Terms applied to the search strategy for Current Contents.
Date Searched: 05.02.19
Date Range of Search: 1970 to Present
N = 497
TS=("Antioxidant TTP" OR "BRN 1888236" OR "Celluflex TPP" OR "DHPF 005" OR "Disflamoll
TP" OR "NSC 57868" OR "O,O,O-Triphenyl phosphate" OR "Phenyl phosphate" OR "Phoscon FR
903N" OR "Phosflex TPP" OR "Phosphoric acid, triphenyl ester" OR "Phosphoric acid, triphenyl ester
radical ion(1+)" OR "Reofos TPP" OR "Sumilizer TPP" OR "Triphenol phosphate" OR
"Triphenoxyphosphine oxide" OR "Triphenyl phosphate" OR "Triphenylphosphat" OR
"Triphenylphosphate" OR "UN 3077" OR "UNII-YZE19Z66EA" OR "Wako TPP" OR "WSFR-TPP")
N = 497
PROQUEST Agricultural and Scientific Database: (www.csa.com)

65
General Search Terms applied to the search strategy for ProQuest Agricultural and Scientific Database.
Date Searched: 05.02.19
Date Range of Search: 1900 to Present
N = 1092
ALL("Antioxidant TTP" OR "BRN 1888236" OR "Celluflex TPP" OR "DHPF 005" OR "Disflamoll
TP" OR "NSC 57868" OR "O,O,O-Triphenyl phosphate" OR "Phenyl phosphate" OR "Phoscon FR
903N" OR "Phosflex TPP" OR "Phosphoric acid, triphenyl ester" OR "Phosphoric acid, triphenyl ester
radical ion(1+)" OR "Reofos TPP" OR "Sumilizer TPP" OR "Triphenol phosphate" OR
"Triphenoxyphosphine oxide" OR "Triphenyl phosphate" OR "Triphenylphosphat" OR
"Triphenylphosphate" OR "UN 3077" OR "UNII-YZE19Z66EA" OR "Wako TPP" OR "WSFR-TPP")
AND STYPE("Scholarly Journals" OR Reports OR Thesis OR "Government Documents") AND
LA(ENG)
N = 1092
PROQUEST Dissertations and Theses: (search.proquest.com)
General Search Terms applied to the search strategy for ProQuest Dissertations and Theses.
Date Searched: 05.03.19
Date Range of Search: 1900 to Present
N = 6
ALL("Antioxidant TTP" OR "BRN 1888236" OR "Celluflex TPP" OR "DHPF 005" OR "Disflamoll
TP" OR "NSC 57868" OR "O,O,O-Triphenyl phosphate" OR "Phenyl phosphate" OR "Phoscon FR
903N" OR "Phosflex TPP" OR "Phosphoric acid, triphenyl ester" OR "Phosphoric acid, triphenyl ester
radical ion(1+)" OR "Reofos TPP" OR "Sumilizer TPP" OR "Triphenol phosphate" OR
"Triphenoxyphosphine oxide" OR "Triphenyl phosphate" OR "Triphenylphosphat" OR
"Triphenylphosphate" OR "UN 3077" OR "UNII-YZE19Z66EA" OR "Wako TPP" OR "WSFR-TPP")
AND LA(ENG)
N = 6
SCIENCE DIRECT: (www.sciencedirect.com)
General Search Terms applied to the search strategy for Science Direct
Date Searched: 05.02.19
Date Range of Search: 1823 to Present
N = 258
Science Direct 01:
"Antioxidant TTP" OR "BRN 1888236" OR "Celluflex TPP" OR "DHPF 005" OR "Disflamoll TP" OR
"NSC 57868" OR "O,O,O-Triphenyl phosphate" OR "Phenyl phosphate" OR "Phoscon FR 903N"
N = 209

66
Science Direct 02:
"Phosflex TPP" OR "Phosphoric acid, triphenyl ester" OR "Phosphoric acid, triphenyl ester radical
ion(1+)" OR "Reofos TPP" OR "Sumilizer TPP" OR "Triphenol phosphate" OR "Triphenoxyphosphine
oxide" OR "Triphenyl phosphate" OR "Triphenylphosphat"
N = 0
Science Direct 03:
"Triphenylphosphate" OR "UN 3077" OR "UNII-YZE19Z66EA" OR "Wako TPP" OR "WSFR-TPP"
N = 49
AGRICOLA: (www.nal.usda.gov)
General Search Terms applied to the search strategy for Agricola. The Agricola database contains a
significant amount of gray literature including proceedings, symposia, and progress reports from
government and educational institutions. Agricola is not used when conducting a search for the Office
of Water.
Date Searched: 05.02.19
Date Range of Search: 15
th
century to the Present
N = 270
Agricola 01:
Antioxidant TTP
BRN 1888236
Celluflex TPP
DHPF 005
Disflamoll TP
NSC 57868
O,O,O-Triphenyl phosphate
Phenyl phosphate
Phoscon FR 903N
Phosflex TPP
N = 56
Agricola 02:
Phosphoric acid, triphenyl ester
Phosphoric acid, triphenyl ester radical ion(1+)
Reofos TPP
Sumilizer TPP
Triphenol phosphate
Triphenoxyphosphine oxide
Triphenyl phosphate
Triphenylphosphat
Triphenylphosphate
UN 3077
67
N = 234
Agricola 03:
UNII-YZE19Z66EA
Wako TPP
WSFR-TPP
N = 0
TOXNET:
(toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?TOXLINE)
General Search Terms applied to the search strategy for TOXNET.
Date Searched: 05.02.19
Date Range of Search: 1900 to Present
N = 624
TOXNET 01:
115-86-6 OR 106971-30-6 OR 402955-02-6
Search
Database
Query
Time
Result
# 2
Toxline
( ( "triphenyl phosphate" OR "phosflex tpp" OR "disflamoll tp"
OR "celluflex tpp" OR 115-86-6 [rn] ) OR 106971-30-6 [rn] OR
402955-02-6 [rn] ) AND ( eng [la] ) AND ( BIOSIS [org] OR
NTIS [org] OR PESTAB [org] OR PubMed [org] OR TSCATS
[org] )
19:35:13
624
N = 624
ECOTOX UNIFY:
This is an internal EPA database that is not accessible to the public. Results from the ECOTOX Unify
search strategy.
Date Searched: 05.03.19
Date Range of Search: all years
N = 162
TPP
N = 162
A.1.2.2 Data Prioritization for Environmental Hazard, Human Health Hazard, Fate
and Physical Chemistry
In brief, SWIFT Review has pre-set literature search strategies (“filters”) developed by information
specialists that can be applied to identify studies that are more likely to be useful for identifying human
health and ecotoxicity content from those that likely do not (e.g., analytical methods). The filters
function like a typical search strategy where studies are tagged as belonging to a certain filter if the
terms in the filter literature search strategy appear in title, abstract, keyword or medical subject headings

68
(MeSH) fields content. The applied SWIFT Review filters focused on lines of evidence: human, animal
models for human health, ecological taxa (which includes ecotoxicological animal models, plants, and
other taxa), and in vitro studies. The details of the search strategies that underlie the filters are available
online. Studies not retrieved using these filters were not considered further. Studies that included one or
more of the search terms in the title, abstract, keyword, or MeSH fields were exported as a RIS file for
screening in Swift-ActiveScreener or DistillerSR
8
.
A.1.2.3 Data Prioritization for Occupational Exposures and Environmental Releases
and General Population, Consumer and Environmental Exposures
To prioritize references related to occupational exposure, environmental release, general population
exposure, consumer exposure, and environmental exposure, EPA used positive and negative seed studies
to build a classification model in SWIFT Review. The positive seeds were identified using relevant
literature pool for the first ten TSCA risk evaluations, while the negative seeds were identified from a
subset of literature for the current high-priority substances. The model was then applied to the
unclassified literature to generate a classification score for each reference. Scores above a certain
threshold value were then prioritized for further review in Swift-ActiveScreener.
Peer-Reviewed Screening Process
The studies identified from publicly available database searches and SWIFT-Review
filtering/prioritization were housed in HERO system and imported into SWIFT-ActiveScreener or
DistillerSR for title/abstract and full-text screening. Both title/abstract and full-text screening were
conducted by two independent reviewers. Screening is initiated with a pilot phase of screening (between
10 and 50) studies to identify areas where clarification in screening criteria might be needed or
chemical-specific supplemental material tags might be identified. Records that met PECO (or equivalent
criteria (A.2.1) during title and abstract screening were considered for full-text screening. At both the
title/abstract and full-text review levels, screening conflicts were resolved by topic-specific experts
and/or discussion among the primary screeners. For citations with no abstract, the articles are initially
screened based on all or some of the following: title relevance (titles that suggest a record is not relevant
can be excluded rather than marked as unclear), and page numbers (articles two pages in length or less
were assumed to be conference reports, editorials, or letters). During title/abstract or full-text level
screening in DistillerSR, studies that did not meet the PECO criteria, but which could provide
supporting information were categorized (or “tagged”) as supplemental information.
It is important to emphasize that being tagged as supplemental material does not mean the study would
necessarily be excluded from consideration in an assessment. The initial screening level distinctions
between a study meeting the PECO criteria and a supplemental study are often made for practical
reasons and the tagging structures (as seen in the literature inventory trees and heat maps in Section 2.1
of this document) are designed to ensure the supplemental studies are categorized for easy retrieval if
needed while conducting the assessment. The impact on the assessment conclusions of individual studies
tagged as supporting material is often difficult to assess during the screening phase of the assessment.
These studies may emerge as being critically important to the assessment and need to be evaluated and
summarized at the individual study level (e.g., cancer MOA mechanistic or non-English-language
studies), or be helpful to provide context (e.g., summarize current levels of exposure, provide hazard
evidence from routes or durations of exposure not pertinent to the PECO), or not be cited at all in the
assessment (e.g., individual studies that contribute to a well-established scientific conclusion). Studies
8
DistillerSR is a web-based systematic review software used to screen studies available at
https://www.evidencepartners.com/products/distillersr-systematic-review-software.

69
maybe be tagged as supplemental material during either title and abstract or full-text screening. When
tagged as supplemental material during title and abstract screening, it may not be completely clear
whether the chemical of interest is reported in the study (i.e., abstracts may not describe all chemicals
investigated). In these cases, studies are still tagged with the expectation that if full-text retrieval is
pursued, then additional screening would be needed to clarify if the study is pertinent.
A.2.1 Inclusion/Exclusion Criteria
A PECO statement is typically used to focus the research question(s), search terms, and
inclusion/exclusion criteria in a systematic review. PECO criteria were developed a priori to screening
and modified to fit the various discipline areas supporting the TSCA risk evaluations. Variations include
the RESO (receptor, exposure, scenario/setting, and outcome) used for the occupational exposure and
environmental releases discipline, and PESO (pathways/processes, exposures, setting/scenario, and
outcomes) used by the fate and transport discipline. All PECOs and PECO-equivalent criteria can be
found in the following sections.
A.2.1.1 PECO for Environmental and Human Health Hazards
The PECO used in this evidence map to identify literature pertinent to TPP effects on human health and
environmental hazard is presented in Table_Apx A-3. In addition to the PECO criteria, studies
containing potentially relevant supplemental material were tracked and categorized during the literature
screening process as outlined in Table_Apx A-4.
Table_Apx A-3. Hazards Title and Abstract and Full-Text PECO Criteria for TPP
PECO
Element
Evidence
P
Human: Any population and life stage (occupational or general population, including
children and other sensitive populations).
Animal: Aquatic and terrestrial species (live, whole organism) of any life stage
(including preconception, in utero, lactation, peripubertal, and adult stages). Include
insects, spiders, amphibians, birds, crustaceans, fish, mollusks, reptiles, worms and
invertebrates. Bacteria and viruses are not included. In most cases, transgenic
animal models will get screened as "yes" or "unclear" at TIAB level.
Plants: Aquatic and terrestrial species (live), all plants including algal, moss, lichen
and fungi species
Screener note:
Mechanistic information including in vitro assays will be tagged as supplemental
material.
E
Relevant forms and isomers:
Triphenyl phosphate (CASRN 115-86-6)
Triphenyl phosphate has a number of synonyms which have been keyword highlighted
in green and should be included.
Triphenyl phosphate has a number of synonyms that can be found on the EPA
Chemistry Dashboard.
70
PECO
Element
Evidence
Other forms should be excluded: phosphoric acid
No isomers were included for TPP.
Human: Any exposure to triphenyl phosphate
Animal: Any exposure to triphenyl phosphate including via water, injection, diet, and
dermal. Studies involving exposures to mixtures will be included only if they
include exposure to triphenyl phosphate alone.
Plants: Exposure to triphenyl phosphate via water or soil, with reported concentration
and duration. Studies involving exposures to mixtures will be included only if they
include exposure to triphenyl phosphate alone. Chemical exposures for aquatic
plants where only sediment concentrations are reported from field studies are
excluded; laboratory-based sediment studies are retained.
C
Human: A comparison or referent population exposed to lower levels (or no
exposure/exposure below detection limits) of triphenyl phosphate, or exposure to
triphenyl phosphate for shorter periods of time. Case reports and case series will be
tracked as “potentially relevant supplemental information.”
Animal and Plants: A concurrent control group exposed to vehicle-only treatment
and/or untreated control (control could be a baseline measurement).
O
Human: All health outcomes (both cancer and noncancer).
Animal and Plants: All biological effects (including bioaccumulation from laboratory
studies with concurrently measured water and tissue concentrations).
Table_Apx A-4. Major Categories of Potentially Relevant Supplemental Material for TPP
Category
Evidence
Mechanistic studies
Studies reporting measurements related to a health outcome that inform the biological or chemical
events associated with phenotypic effects, in both mammalian and non-mammalian model systems,
including in vitro, in vivo (by various non-inhalation routes of exposure), ex vivo, and in silico
studies.
ADME, PBPK, and
toxicokinetic
Studies designed to capture information regarding absorption, distribution, metabolism, and excretion
(ADME), toxicokinetic studies, or physiologically based pharmacokinetic (PBPK) models.
Note: Exposure studies with biomonitoring or biomarker information (e.g. TPP metabolites in blood or
urine) are considered ADME. Environmental exposure studies (e.g. TPP in dust) are EXCLUDED.
Susceptible
populations
(no health outcome)
Studies that identify potentially susceptible subgroups; for example, studies that focus on a specific
demographic, life stage, or genotype.
Mixture studies
Mixture studies that are not considered PECO-relevant because they do not contain an exposure or
treatment group assessing only the chemical of interest.
Case reports or case
series
Case reports (n ≤ 3 cases) and case series/studies (<20 cases) will be tracked as potentially relevant
supplemental information.
Non-English records
Non-English records will be tracked as potentially relevant supplemental information.

71
Category
Evidence
Records with no
original data
Records that do not contain original data, such as other agency assessments, informative scientific
literature reviews, editorials or commentaries.
Conference abstracts
Records that do not contain sufficient documentation to support study evaluation and data extraction.
TPP used as a
synergist
TPP used as synergist with unbounded NOEC/NOAEL. For example, the text notes that no mortality
was found at doses TPP was administered.
A.2.1.2 PECO for Consumer, Environmental, and General Population Exposures
Table_Apx A-5. Generic Inclusion Criteria for the Data Sources Reporting Exposure Data on
General Population, Consumers and Environmental Receptors
PECO Element
Evidence
Population
Human: General population; consumers; bystanders in the home; near-facility populations
(includes industrial and commercial facilities manufacturing, processing, or using the chemical
substance); children; susceptible populations (life stages, preexisting conditions, genetic
factors), pregnant women; lactating women, women of child-bearing age. Many human
population groups may be exposed. No chemical-specific exclusions are suggested at this time.
Environmental: aquatic species, terrestrial species, terrestrial plants, aquatic plants (field
studies only)
Exposure
Expected Primary Exposure Sources, Pathways, Routes:
Pathways: indoor air/vapor/mist; indoor dust; particles; outdoor/ambient air; surface water;
biosolids; sediment; breastmilk; food items containing TPP including fish; consumer product
uses in the home (including consumer product containing chemical);
Routes of Exposure: Inhalation, Oral, Dermal
Comparator
(Scenario)
Human: Consider media-specific background exposure scenarios and use/source specific
exposure scenarios as well as which receptors are and are not reasonably exposed across the
projected exposure scenarios.
Environmental Consider media-specific background exposure scenarios and use/source
specific exposure scenarios as well as which receptors are and are not reasonably exposed
across the projected exposure scenarios.
Outcomes for
Exposure
Concentration or
Dose
Human: Acute, subchronic, and/or indoor air and water concentration estimates (mg/m
3
or
mg/L). Both external potential dose and internal dose based on biomonitoring and reverse
dosimetry mg/kg/day will be considered. Characteristics of consumer products or articles
(weight fraction, emission rates, etc) containing TPP.
Environmental: A wide range of ecological receptors will be considered (range depending on
available ecotoxicity data) using surface water concentrations, sediment concentrations.
72
Table_Apx A-6. Pathways Identified as Supplemental for TPP
a
Chemical
Drinking
Water
Ambient Air
Air
Disposal
Land
Disposal
Underground
Disposal
Ground Water
Phosphoric acid,
triphenyl ester
(TPP)
--
--
--
--
--
--
a
“Supplemental pathways” refer to pathways addressed by other EPA administered statutes.
Studies tagged under these pathways provide media information that is not prioritized in the screening process.
A.2.1.3 RESO for Occupational Exposure and Environmental Releases
EPA developed a generic RESO statement to guide the screening of engineering and occupational
exposure data or information sources for the TSCA risk evaluations. Data or information sources that
comply with the inclusion criteria specified in the RESO statement are eligible for inclusion, considered
for evaluation, and possibly included in the environmental release and occupational exposure
assessments. On the other hand, data or information sources that fail to meet the criteria in the RESO
statement are excluded from further consideration.
Assessors seek information on various chemical-specific engineering and occupational exposure data
needs as part of the process of developing the exposure assessment for each risk evaluation. EPA uses
the RESO statement (Table_Apx A-7.) along with the information in Table_Apx A-8. when screening
the engineering and occupational exposure data and information.
Table_Apx A-7. Inclusion Criteria for Data Sources Reporting Engineering and Occupational
Exposure Data
RESO
Element
Evidence
Receptors
• Humans:
Workers, including occupational non-users
• Environment:
All environmental receptors (relevant release estimates input to Exposure)
Please refer to the conceptual models for more information about the environmental
and human receptors included in the TSCA risk evaluation.
Exposure
• Worker exposure to and relevant environmental releases of the chemical
substance from occupational scenarios:
Dermal and inhalation exposure routes (as indicated in the conceptual model)
Oral route (as indicated in the conceptual model)
Please refer to the conceptual models for more information about the routes and
media/pathways included in the TSCA risk evaluation.
Setting or
Scenario
• Any occupational setting or scenario resulting in worker exposure and relevant
environmental releases (includes all manufacturing, processing, use, disposal.
73
RESO
Element
Evidence
Outcomes
• Quantitative estimates* of worker exposures and of relevant environmental
releases from occupational settings
• General information and data related and relevant to the occupational estimates*
* Metrics (e.g., mg/kg/day or mg/m
3
for worker exposures, kg/site/day for releases) are determined by toxicologists for
worker exposures and by exposure assessors for releases; also, the Engineering, Release and Occupational Exposure Data
Needs (Table_Apx A-8) provides a list of related and relevant general information.
TSCA=Toxic Substances Control Act
Table_Apx A-8. Engineering, Environmental Release and Occupational Data Necessary to
Develop the Environmental Release and Occupational Exposure Assessments
Objective
Determined
during Scoping
Type of Data
a
General
Engineering
Assessment (may
apply to
Occupational
Exposures and /
or Environmental
Releases)
Description of the life cycle of the chemical(s) of interest, from manufacture to end-of-life (e.g.,
each manufacturing, processing, or use step), and material flow between the industrial and
commercial life cycle stages.
The total annual U.S. volume (lb/yr or kg/yr) of the chemical(s) of interest manufactured,
imported, processed, and used; and the share of total annual manufacturing and import volume
that is processed or used in each life cycle step.
Description of processes, equipment, and unit operations during each industrial/ commercial life
cycle step.
Material flows, use rates, and frequencies (lb/site-day or kg/site-day and days/yr; lb/site-batch and
batches/yr) of the chemical(s) of interest during each industrial/ commercial life cycle step. Note:
if available, include weight fractions of the chemicals (s) of interest and material flows of all
associated primary chemicals (especially water).
Number of sites that manufacture, process, or use the chemical(s) of interest for each industrial/
commercial life cycle step and site locations.
Concentration of the chemical of interest
Occupational
Exposures
Description of worker activities with exposure potential during the manufacture, processing, or use
of the chemical(s) of interest in each industrial/commercial life cycle stage.
Potential routes of exposure (e.g., inhalation, dermal).
Physical form of the chemical(s) of interest for each exposure route (e.g., liquid, vapor, mist) and
activity.
Breathing zone (personal sample) measurements of occupational exposures to the chemical(s) of
interest, measured as time-weighted averages (TWAs), short-term exposures, or peak exposures
in each occupational life cycle stage (or in a workplace scenario similar to an occupational life
cycle stage).
Area or stationary measurements of airborne concentrations of the chemical(s) of interest in each
occupational setting and life cycle stage (or in a workplace scenario similar to the life cycle stage
of interest).
For solids, bulk and dust particle size characterization data.
Dermal exposure data.
Exposure duration (hr/day).
Exposure frequency (days/yr).
Number of workers who potentially handle or have exposure to the chemical(s) of interest in each
occupational life cycle stage.
Personal protective equipment (PPE) types employed by the industries within scope.
74
Objective
Determined
during Scoping
Type of Data
a
Engineering controls employed to reduce occupational exposures in each occupational life cycle
stage (or in a workplace scenario similar to the life cycle stage of interest), and associated data or
estimates of exposure reductions.
Environmental
Releases (to
relevant
environmental
media)
Description of sources of potential environmental releases, including cleaning of residues from
process equipment and transport containers, involved during the manufacture, processing, or use
of the chemical(s) of interest in each life cycle stage.
Estimated mass (lb or kg) of the chemical(s) of interest released from industrial and commercial
sites to each environmental medium (water) and treatment and disposal methods (POTW),
including releases per site and aggregated over all sites (annual release rates, daily release rates)
Release or emission factors.
Number of release days per year.
Waste treatment methods and pollution control devices employed by the industries within scope
and associated data on release/emission reductions.
a
These are the tags included in the full-text screening form. The screener makes a selection from these specific tags,
which describe more specific types of data or information.
In addition to the data types listed above, EPA may identify additional data needs for mathematical modeling. These
data needs will be determined on a case-by-case basis.
Abbreviations:
hr=Hour
kg=Kilogram(s)
lb=Pound(s)
yr=Year
PV=Particle volume
POTW=Publicly owned treatment works
PPE=Personal protection equipment
PSD=Particle size distribution
TWA=Time-weighted average
A.2.1.4 PESO for Fate and Transport
EPA developed a generic PESO statement to guide the screening of environmental fate data or
information sources for the TSCA risk evaluations. Data or information sources that comply with the
inclusion criteria in the PESO statement are eligible for inclusion, considered for evaluation, and
possibly included in the environmental fate assessment. On the other hand, data or information sources
that fail to meet the criteria in the PESO statement are excluded from further consideration.
Assessors seek information on various chemical-specific fate endpoints and associated fate processes,
environmental media and exposure pathways as part of the process of developing the environmental fate
assessment for each risk evaluation. EPA uses the PESO statement (Table_Apx A-9.) along with the
information in Table_Apx A-10 when screening the fate data or information sources to ensure complete
coverage of the processes, pathways and data or information relevant to the environmental fate and
transport of the chemical substance undergoing risk evaluation.
75
Table_Apx A-9. Inclusion Criteria for Data or Information Sources Reporting Environmental
Fate and Transport Data
PESO
Element
Evidence
Pathways and Processes
Environmental fate, transport, partitioning and degradation behavior across
environmental media to inform exposure pathways of the chemical substance of
interest
Exposure pathways included in the conceptual models: air, surface water, groundwater,
wastewater, soil, sediment and biosolids.
Processes associated with the target exposure pathways
Bioconcentration and bioaccumulation
Destruction and removal by incineration
Please refer to the conceptual models for more information about the exposure pathways
included in each TSCA risk evaluation.
Exposure
Environmental exposure of environmental receptors (i.e., aquatic and terrestrial
organisms) to the chemical substance of interest, mixtures including the chemical
substance, and/or its degradation products and metabolites
Environmental exposure of human receptors, including any potentially exposed or
susceptible subpopulations, to the chemical substance of interest, mixtures including
the chemical substance, and/or its degradation products and metabolites
Please refer to the conceptual models for more information about the environmental and
human receptors included in each TSCA risk evaluation.
Setting or Scenario
Any setting or scenario resulting in releases of the chemical substance of interest into the
natural or built environment (e.g., buildings including homes or workplaces, or wastewater
treatment facilities) that would expose environmental (i.e., aquatic and terrestrial
organisms) or human receptors (i.e., general population, and potentially exposed or
susceptible subpopulation)
Outcomes
Fate properties which allow assessments of exposure pathways:
Abiotic and biotic degradation rates, mechanisms, pathways, and products
Bioaccumulation magnitude and metabolism rates
Partitioning within and between environmental media (see Pathways and Processes)
Table_Apx A-10. Fate Endpoints and Associated Processes, Media and Exposure Pathways
Considered in the Development of the Environmental Fate Assessment
Fate Data Endpoint
Associated Process(es)
Associated Media/Exposure Pathways
Surface
Water,
Wastewater,
Sediment
Soil,
Biosolids
Groundwater
Air
Required Environmental Fate Data
Abiotic reduction rates or half-
lives
Abiotic reduction,
Abiotic dehalogenation
X
Aerobic biodegradation rates
or half-lives
Aerobic biodegradation
X
X
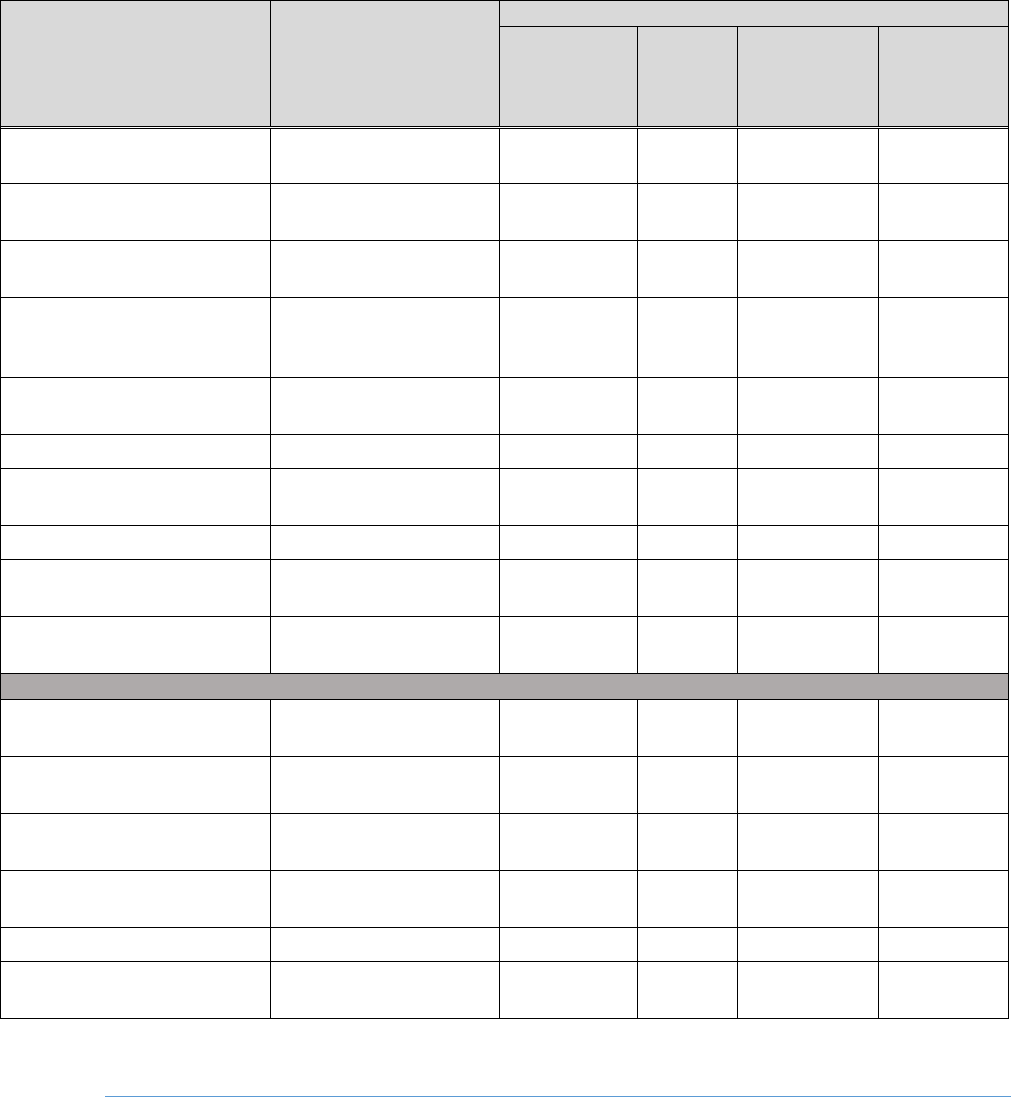
76
Fate Data Endpoint
Associated Process(es)
Associated Media/Exposure Pathways
Surface
Water,
Wastewater,
Sediment
Soil,
Biosolids
Groundwater
Air
Anaerobic biodegradation
rates or half-lives
Anaerobic
biodegradation
X
X
X
Aqueous photolysis (direct
and indirect) rates or half-lives
Aqueous photolysis
(direct and indirect)
X
Atmospheric photolysis (direct
and indirect) rates or half-lives
Atmospheric photolysis
(direct and indirect)
X
Bioconcentration factor
(BCF), Bioaccumulation
factor (BAF)
Bioconcentration,
Bioaccumulation
X
X
X
Biomagnification and related
information
Trophic magnification
X
X
Desorption information
Sorption, Mobility
X
X
X
Destruction and removal by
incineration
Incineration
X
Hydrolysis rates or half-lives
Hydrolysis
X
X
X
K
OC
and other sorption
information
Sorption, Mobility
X
X
X
Wastewater treatment removal
information
Wastewater treatment
X
X
Supplemental (or Optional) Environmental Fate Data
Abiotic transformation
products
Hydrolysis, Photolysis,
Incineration
X
X
Aerobic biotransformation
products
Aerobic biodegradation
X
X
Anaerobic biotransformation
products
Anaerobic
biodegradation
X
X
X
Atmospheric deposition
information
Atmospheric deposition
X
Coagulation information
Coagulation, Mobility
X
X
Incineration removal
information
Incineration
X
A.2.1.5 Generation of Hazard Heat Maps
As stated in Appendix A.1.2.2, SWIFT Review has pre-set literature search strategies (“filters”)
developed by information specialists that can be applied to identify studies that are more likely to be
useful for identifying human health and ecotoxicity content. The filters function like a typical search
strategy where studies are tagged as belonging to a certain filter if the terms in the filter literature search
strategy appear in title, abstract, keyword or MeSH fields content.
After the completion of full-text screening for hazard data, all references tagged as included (or
“PECO-relevant) were uploaded to the SWIFT Review tool for further filtering. The SWIFT

77
Review filters applied at this phase focused on types of health outcomes included: “ADME”,
“PBPK”, “cancer”, “cardiovascular”, “developmental”, “endocrine”, “gastrointestinal”,
“hematological and immune”, “hepatic”, “mortality”, “musculoskeletal”, “neurological”,
“nutritional and metabolic”, “ocular and sensory”, “renal”, “reproductive”, “respiratory”, and
“skin and connective tissue”. The details of these health outcome search strategies that underlie
the filters are available online. Studies that included one or more of the search terms in the title,
abstract, keyword, or MeSH fields were exported and used to populate the Hazard Heat Map (
Figure 2-10). Studies that were not retrieved using these filters were tagged as “No Tag”. The evidence
type listed in the heat map (e.g., human, animal-human health model, animal- environmental model, and
plant) was manually assigned to each reference by screeners during the full-text screening.
The health outcome tags were originally designed for vertebrate systems, and as such, did not conform
well to plant evidence. Therefore, any plant studies tagged for: “cancer”, “cardiovascular”,
“gastrointestinal”, “hematological and immune”, “hepatic”, “musculoskeletal”, “neurological”, “ocular
and sensory” and “renal and respiratory” were manually reviewed and re-tagged to more appropriate
health outcomes.
Gray Literature Search and Screening Strategies
EPA conducted a gray literature search for available information to support the TSCA risk evaluations
for the next twenty TSCA risk evaluations. Gray literature is defined as the broad category of
data/information sources not found in standard, peer-reviewed literature databases (e.g., PubMed and
Web of Science). Gray literature includes data/information sources such as white papers, conference
proceedings, technical reports, reference books, dissertations, information on various stakeholder
websites, and other databases. Given the nature of how gray literature is searched and collected, results
may not come with a bibliographic citation or abstract and were therefore processed using a decision
tree logic described in Appendix A.3.1 for potential relevance prior to entering full text screening where
a discipline-specific PECO is applied.
Search terms were variable dependent on source and based on knowledge of a given source to provide
discipline-specific information. A summary of sources is provided in Appendix A.3.4. The criteria for
determining the potential relevance of documents identified from gray literature sources is described in
the following sections for each discipline.
A.3.1 Screening of Gray Literature
To reduce the overall burden of processing gray literature results, EPA developed a screening process to
determine the potential relevance of gray literature. This step was introduced prior to collecting the
resulting documents. Figure_Apx A-1 describes the decision logic used to screen gray literature results.
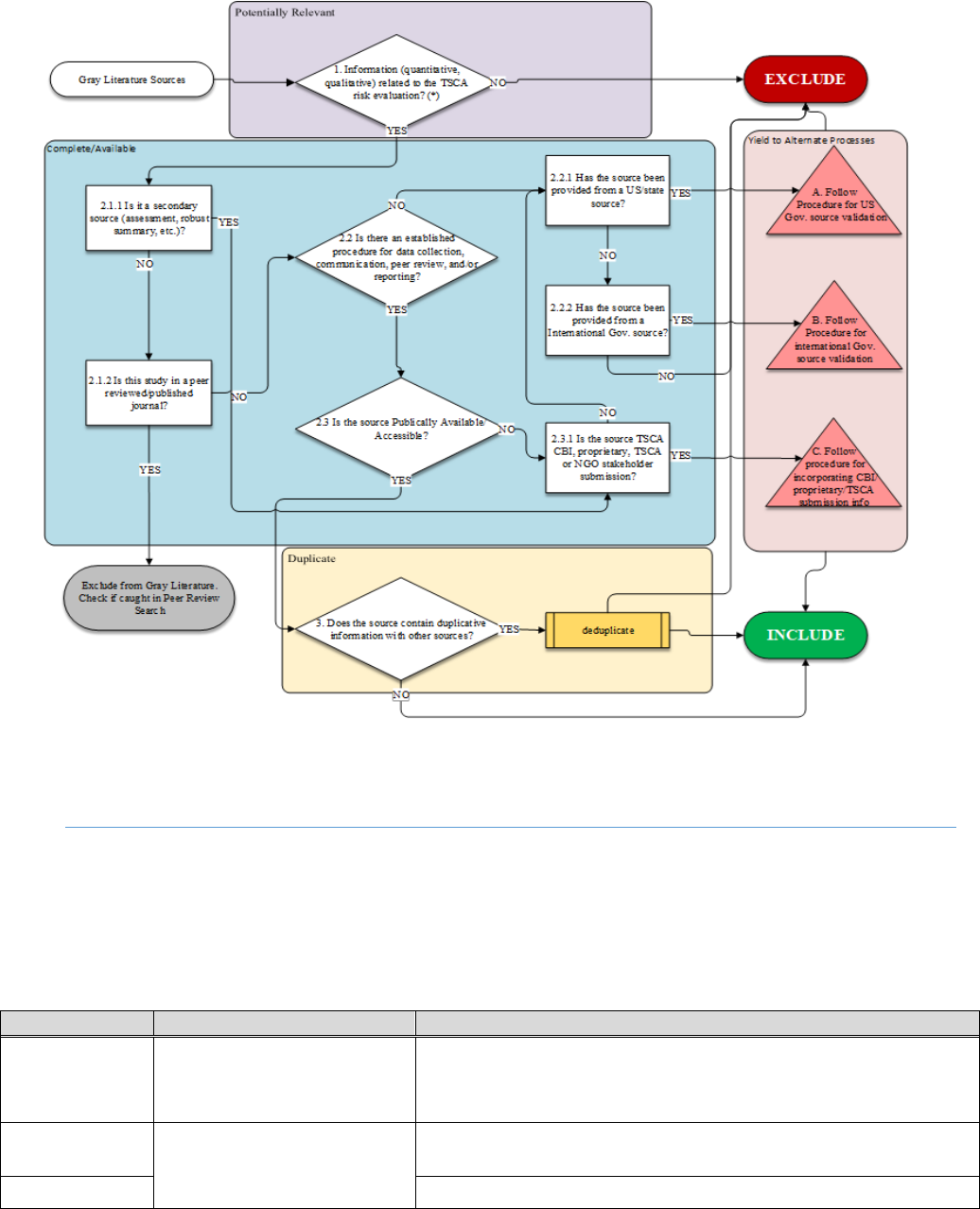
78
Figure_Apx A-1. Decision Logic Tree Used to Screen Gray Literature Results
A.3.2 Initial Screening of Sources using Decision Logic Tree
The purpose of the inclusion/exclusion decision logic tree in Figure_Apx A-1 is to provide a broad,
general screening technique to determine whether each gray literature source should be included and
further screened or excluded with no additional screening necessary. The diamonds in the decision tree
require analysis by the screener, whereas the rectangular boxes are used to classify the type of source.
All the questions used in the decision process are provided in Table_Apx A-11.
Table_Apx A-11. Decision Logic Tree Overview
Step
Metric
Questions to Consider
1
Potential Relevance
Does the result have information (qualitative or quantitative) related to
TSCA risk evaluations?
*Apply Discipline relevancy metric
2.1.1
Complete / Available
Is it a secondary data source (assessment, robust summary, TSCA
submission databases, etc.)?
2.1.2
Is the document from a peer reviewed/published journal?
79
Step
Metric
Questions to Consider
2.2
Is there an established procedure for data collection, communication,
peer review, and/or reporting?
2.2.1
Has the data been provided by a US governmental/state source?
2.2.2
Has the data been provided by an international governmental source?
2.3
Are these data publicly available/accessible?
2.3.1
Is the source TSCA CBI, proprietary, TSCA or NGO stakeholder
submission?
3
Duplicate
Does the result contain any duplicative information found in other
sources?
Results of the gray literature search and decision tree process are included in Appendix A.3.4.
A.3.3 TSCA Submission Searching and Title Screening
EPA screens information submitted under TSCA Sections 4, 5, 8(e), and 8(d), as well as for your
information (FYI) submissions. In the gray literature process defined in Appendix A.3.2, EPA considers
the databases that contain TSCA submissions to be secondary sources (Step 1.1) because the metadata in
the databases are secondary. These databases then advance to Step 2.3.1 and then to Process C. The
Process C steps are described here.
EPA first screens the titles using two screeners per title. EPA conducts this step primarily to reduce the
number of full studies to be obtained because some studies are available only on microfiche or in long-
term storage. Screening is done using the inclusion and exclusion criteria within the relevant PECOs,
PESOs or RESOs for each topic area (Appendix A.2.1). EPA excludes interim reports (e.g., interim
sacrifices for toxicity studies) and only final reports are further considered. If the title is not clear
regarding the document’s contents, EPA obtains the full text and advances to the next steps.
After full texts are obtained, EPA reviewed some sources (prior to full-text screening) based on whether
they have several factors; primary data, an established procedure for peer review, data collection,
communication and/or reporting and are publicly available. Sources that have these factors will move on
to full text screening. Other sources will go straight to full text screening using PECO-type criteria
without going through this extra step.
EPA may decide to initiate a backwards search on sources that are deemed to have secondary data. In
situations where parameters such as procedures for peer review and data collection are unclear, EPA
may reach out to the authors to retrieve information to gauge whether the source should be included or
excluded. Studies that are not publicly available (such as proprietary or CBI sources) may undergo
additional screening steps.
During the full-text screening step, two individuals screen each source according to the PECOs, PESOs
and RESOs (Appendix A.2.1).
Results of the TSCA submission search and decision tree process are included in Appendix A.3.4.
80
A.3.4 Gray Literature Search Results for TPP
Table_Apx A-12. provides a list of gray literature sources that yielded results for TPP.
Table_Apx A-12. Gray Literature Sources that Yielded Results for TPP
Source
Agency
Source Name
Source Type
Source
Category
Source Website
ATSDR
ATSDR Toxicological
Profiles (original publication)
Other US
Agency
Resources
Assessment
or Related
Document
https://www.atsdr.cdc.gov/tox
profiles/index.asp
Australian
Government,
Department
of Health
NICNAS Assessments
(human health, Tier I, II or
III)
International
Resources
Assessment
or Related
Document
https://www.industrialchemic
als.gov.au/chemical-
information/search-
assessments
CPSC
Technical Reports:
Exposure/Risk Assessment
Other US
Agency
Resources
Assessment
or Related
Document
https://www.cpsc.gov/Resear
ch--Statistics/Chemicals
ECHA
ECHA Documents
International
Resources
Assessment
or Related
Document
https://echa.europa.eu/inform
ation-on-chemicals
EPA
OPPT: TSCATS database
maintained at SRC (TSCA
submissions)
US EPA
Resources
Database
EPA
OPPT: Chemview (TSCA
submissions - chemical test
rule data and substantial risk
reports)
US EPA
Resources
Database
https://chemview.epa.gov/che
mview
EPA
OPPT: CIS (CBI LAN)
(TSCA submissions)
US EPA
Resources
Database
EPA
Office of Air: National
Emissions Inventory (NEI) -
National Emissions Inventory
(NEI) Data (2014, 2011,
2008)
US EPA
Resources
Database
https://www.epa.gov/air-
emissions-inventories/2014-
national-emissions-inventory-
nei-data
EPA
Office of Water: STORET
and WQX
US EPA
Resources
Database
https://www.waterqualitydata
.us/portal/
EPA
Design for the Environment
(DfE) Alternatives
Assessments
US EPA
Resources
Assessment
or Related
Document
https://www.epa.gov/safercho
ice/design-environment-
alternatives-assessments
EPA
Other EPA: Misc sources
US EPA
Resources
General
Search
https://www.epa.gov/
EPA
EPA: AP-42
US EPA
Resources
Regulatory
Document or
List
https://www.epa.gov/air-
emissions-factors-and-
quantification/ap-42-
compilation-air-emissions-
factors
81
Source
Agency
Source Name
Source Type
Source
Category
Source Website
EPA
Office of Water: CFRs
US EPA
Resources
Regulatory
Document or
List
https://www.epa.gov/eg
EPA
Office of Air: CFRs and
Dockets
US EPA
Resources
Regulatory
Document or
List
https://www.epa.gov/stationar
y-sources-air-pollution
EPA
EPA: Generic Scenario
US EPA
Resources
Assessment
or Related
Document
https://www.epa.gov/tsca-
screening-tools/chemsteer-
chemical-screening-tool-
exposures-and-
environmental-
releases#genericscenarios
Japan
Japanese Ministry of the
Environment Assessments -
Environmental Risk
Assessments
International
Resources
Assessment
or Related
Document
https://www.env.go.jp/en/che
mi/prtr/substances/
ILO
International Chemical Safety
Cards (ICSCs)
International
Resources
Database
https://www.ilo.org/safework/
info/publications/WCMS_11
3134/lang--en/index.htm
KOECT
Kirk-Othmer Encyclopedia of
Chemical Technology Journal
Article
Other
Resource
Encyclopedia
https://onlinelibrary.wiley.co
m/doi/book/10.1002/0471238
961
NIOSH
CDC NIOSH - Occupational
Health Guideline Documents
Other US
Agency
Resources
Assessment
or Related
Document
https://www.cdc.gov/niosh/in
dex.htm
NIOSH
CDC NIOSH - Pocket Guide
Other US
Agency
Resources
Database
https://www.cdc.gov/niosh/np
g/default.html
NIOSH
CDC NIOSH - Health Hazard
Evaluations (HHEs)
Other US
Agency
Resources
Assessment
or Related
Document
https://www2a.cdc.gov/hhe/s
earch.asp
NIOSH
CDC NIOSH - Publications
and Products
Other US
Agency
Resources
Assessment
or Related
Document
https://www2a.cdc.gov/niosht
ic-2/
NTP
Additional NTP Reports
Other US
Agency
Resources
Assessment
or Related
Document
https://ntp.niehs.nih.gov/publi
cations/index.html
OECD
OECD SIDS
International
Resources
Assessment
or Related
Document
https://hpvchemicals.oecd.org
/ui/Publications.aspx
OECD
OECD Substitution and
Alternatives Assessment
International
Resources
Assessment
or Related
Document
http://www.oecdsaatoolbox.o
rg/
82
Source
Agency
Source Name
Source Type
Source
Category
Source Website
OECD
OECD Emission Scenario
Documents
International
Resources
Assessment
or Related
Document
http://www.oecd.org/docume
nt/46/0,2340,en_2649_20118
5_2412462_1_1_1_1,00.html
OECD
OECD: General Site
International
Resources
General
Search
https://www.oecd.org/
OSHA
OSHA Chemical Exposure
Health Data
Other US
Agency
Resources
Database
https://www.osha.gov/openg
ov/healthsamples.html
RIVM
RIVM Reports: Risk
Assessments
International
Resources
Assessment
or Related
Document
https://www.rivm.nl/en
TERA
Toxicology Excellence for
Risk Assessment
Other
Resources
Assessment
or Related
Document
http://www.tera.org/
83
PHYSICAL AND CHEMICAL PROPERTIES
Table_Apx B-1 summarizes statistics for the physical and chemical property values identified through
systematic review as of June 2020. The “N” column indicates the number of unique primary sources of
data for that endpoint. That is, if multiple sources presented equivalent values and cited the same
primary source, only one of those was included in these statistics and included in the statistical
calculations. All physical and chemical property values that were extracted and evaluated as of June
2020 are presented in the supplemental file Data Extraction and Data Evaluation Tables for Physical
and Chemical Property Studies (EPA-HQ-OPPT-2018-0451).
Table_Apx B-1. Summary Statistics for Reviewed Physical Properties
Property or Endpoint
N
Unit
Mean
Standard
Deviation
Min
Max
Molecular formula
-
-
NA
NA
NA
NA
Molecular weight
-
g/mol
NA
NA
NA
NA
Physical state
4
-
NA
NA
NA
NA
Physical properties
7
-
NA
NA
NA
NA
Melting point
22
ºC
49.7
1.1
47.5
52
Boiling point
9
ºC
324
94.7
245
452
Density
4
g/cm
3
1.232
0.066
1.185
1.33
Vapor pressure
2
mm Hg
4.14 × 10
-6
3.03 × 10
-6
2.00 × 10
-6
6.28 × 10
-6
Vapor density
1
-
1.19
-
1.19
1.19
Water solubility
2
mg/L
1.32
0.83
0.73
1.9
Octanol/water partition
coefficient (log Kow)
3
-
4.63
0.061
4.59
4.7
Henry’s Law constant
0
atm·m
3
/mol
-
-
-
-
Flash point
3
ºC
222
1.73
220
223
Auto flammability
0
ºC
-
-
-
-
Viscosity
0
cP
-
-
-
-
Refractive index
1
-
1.55
-
1.55
1.55
Dielectric constant
0
-
-
-
-
-
NA = Not applicable
84
ENVIRONMENTAL FATE AND TRANSPORT
PROPERTIES
Table Apx C-1 provides the environmental fate characteristics that EPA identified and considered in
developing the scope for triphenyl phosphate. This information was presented in the Proposed
Designation of Triphenyl Phosphate (CASRN 115-86-6) as a High-Priority Substance for Risk
Evaluation (U.S. EPA, 2019d) and may be updated as EPA collects additional information through
systematic review methods.
Table_Apx C-1 Environmental Fate and Transport Properties of TPP
Property or Endpoint
Value
a
Reference
Direct Photodegradation
Not expected to be susceptible to direct
photolysis by sunlight because the
chemical does not absorb light at
wavelengths >290 nm
HSDB (2019)
Indirect Photodegradation
t
1/2
= 12 hours
(based on OH reaction rate constant of
1.11 × 10
-11
cm
3
/mol·second at 25 ºC
and 5 × 10
5
OH radicals/cm
3
;
estimated)
b
HSDB (2019) citing EPI Suite
U.S. EPA (2012b)
Hydrolysis
t
1/2
= 19 days (pH 7 at 25 ºC) t
1/2
= 3
days (pH 9 at 25 ºC)
HSDB (2019) citing Mayer et
al. (1981)
t
1/2
= 7.5 days (pH 8.2 at 21 ºC) t
1/2
=
1.3 days (pH 9.5 at 21 ºC)
HSDB (2019) citing Howard
and Deo (1979)
Biodegradation (Aerobic)
t
1/2
= 2–4 days in river die-away tests
(Mississippi River)
HSDB (2019) citing Saeger
and Kaley (1979)
48% mineralization/32 days; t
1/2
= 37
days (loamy sand)
HSDB (2019) citing Anderson
et al. (1993)
100%/7–8 days (freshwater)
HSDB (2019) citing Howard
and Deo (1979)
83–94%/4 weeks based on BOD
(Japanese MITI test)
HSDB (2019) citing NITE
(2019)
Biodegradation
(Anaerobic)
t
1/2
= 32 days (loamy sand)
HSDB (2019) citing Anderson
et al. (1993)
Wastewater Treatment
61% total removal (0.56% by
biodegradation, 60% by sludge and
0.07% by volatilization to air;
estimated)
b
EPI Suite U.S. EPA (2012b)
Bioconcentration Factor
180–280 (Salmo gairdneri) for Pydraul
50E, a hydraulic fluid containing 35%
TPP
HSDB (2019) citing Lombardo
and Egry (1979)
132–364 (Oncorhynchus mykiss)
HSDB (2019) citing Mayer et
al. (1981)
573 (Oncorhynchus mykiss); 561
(Pimephales promelas)
HSDB (2019) citing Muir et al.
(1983)
Bioaccumulation Factor
73 (estimated)
b
EPI Suite U.S. EPA (2012b)

85
Property or Endpoint
Value
a
Reference
Soil Organic
Carbon:Water Partition
Coefficient (Log K
OC
)
3.40, 3.55, and 3.44 (silty clay, loamy
sand, and silt loam, respectively)
HSDB (2019) citing Anderson
et al. (1993)
a
Measured unless otherwise noted
b
EPI SuiteTM physical property inputs: Log Kow = 4.59, MP = 50.5 ºC, VP = 6.4 × 10-6 mm Hg, WS =
1900 mg/L. SMILES:O=P(Oc(cccc1)c1)(Oc(cccc2)c2)Oc(cccc3)c3
OH = hydroxyl radical; BOD = biological oxygen demand; MITI = Ministry of International Trade
and Industry
86
REGULATORY HISTORY
The chemical substance, TPP, is subject to federal and state laws and regulations in the United States
(Table_Apx D-1 and Table_Apx D-2). Regulatory actions by other governments, tribes and
international agreements applicable to TPP are listed in Table_Apx D-3.
Federal Laws and Regulations
Table_Apx D-1 Federal Laws and Regulations
Statutes/Regulations
Description of Authority/Regulation
Description of Regulation
EPA Statutes/Regulations
Toxic Substances
Control Act (TSCA) –
Section 6(b)
EPA is directed to identify high-
priority chemical substances for risk
evaluation; and conduct risk
evaluations on at least 20 high priority
substances no later than three and one-
half years after the date of enactment of
the Frank R. Lautenberg Chemical
Safety for the 21st Century Act.
TPP is one of the 20 chemicals
EPA designated as a High-
Priority Substance for risk
evaluation under TSCA (84 FR
71924, December 30, 2019).
Designation of TPP as a high-
priority substance constitutes the
initiation of the risk evaluation
on the chemical.
Toxic Substances
Control Act (TSCA) –
Section 8(a)
The TSCA Section 8(a) CDR Rule
requires manufacturers (including
importers) to give EPA basic exposure-
related information on the types,
quantities and uses of chemical
substances produced domestically and
imported into the United States.
TPP manufacturing (including
importing), processing and use
information is reported under
the CDR rule (76 FR 50816,
August 16, 2011).
Toxic Substances
Control Act (TSCA) –
Section 8(b)
EPA must compile, keep current and
publish a list (the TSCA Inventory) of
each chemical substance manufactured
(including imported) or processed in
the United States.
TPP was on the initial TSCA
Inventory and therefore was not
subject to EPA’s new chemicals
review process under TSCA
Section 5 (60 FR 16309, March
29, 1995).
Toxic Substances
Control Act (TSCA) –
Section 8(e)
Manufacturers (including importers),
processors, and distributors must
immediately notify EPA if they obtain
information that supports the
conclusion that a chemical substance or
mixture presents a substantial risk of
injury to health or the environment.
EPA received one Substantial
Risk Report for TPP (1992).
https://chemview.epa.gov/chemvie
w
87
Statutes/Regulations
Description of Authority/Regulation
Description of Regulation
Toxic Substances
Control Act (TSCA) –
Section 4
Provides EPA with authority to issue
rules and orders requiring
manufacturers (including importers)
and processors to test chemical
substances and mixtures.
EPA received 67 studies
including ecotox, environmental
fate, human health, and physical
and chemical properties.
(https://chemview.epa.gov/chemvie
w,
Other Federal Statutes/Regulations
Occupational Safety
and Health Act
(OSHA)
Requires employers to provide their
workers with a place of employment
free from recognized hazards to safety
and health, such as exposure to toxic
chemicals, excessive noise levels,
mechanical dangers, heat or cold stress
or unsanitary conditions (29 U.S.C
Section 651 et seq.). Under the Act,
OSHA can issue occupational safety
and health standards including such
provisions as Permissible Exposure
Limits (PELs), exposure monitoring,
engineering and administrative control
measures, and respiratory protection.
In 1970, OSHA issued
occupational safety and health
standards for TPP that included
a PEL of TWA of
3 mg/m
3.
and respirator
recommendations. (29 CFR
1910.1000).
State Laws and Regulations
Table_Apx D-2. State Laws and Regulations
State Actions
Description of Action
State Prohibitions
California adopted a prohibition, effective on January 1, 2020, on the
selling and distribution in commerce of new, not previously owned
juvenile products, mattresses, or upholstered furniture that contains, or
a constituent component of which contains, covered flame retardant
chemicals at levels above 1,000 parts per million (A.B. 2998,
Legislative Council, Sess. 2017-2018, C.A. 2018)
https://legiscan.com/CA/text/AB2998/id/1774418
State PELs
California (PEL of 3 mg/m
3
)
(Cal Code Regs. Title 8, § 5155)
https://www.dir.ca.gov/Title8/5155table_ac1.html
Hawaii (PEL- TWA of 3 mg/m
3)
and STEL (6 mg/m
3)
(Hawaii
Administrative Rules section 12-60-50)
https://labor.hawaii.gov/hiosh/files/2012/12/12-60-General-Safety-
Health-Requirements.pdf

88
State Actions
Description of Action
Minnesota (PEL of 3mg/m
3
) (MNOSHA Permissible Exposure
Limits- Limits for Air Contaminants)
https://www.dli.mn.gov/sites/default/files/pdf/pels.pdf
State Right-to-Know Acts
Massachusetts (105 Code Mass. Regs. § 670.000 Appendix A)
https://www.mass.gov/files/documents/2017/09/11/105cmr670.pdf
New Jersey (N.J.A.C. 7:1C)
http://web.doh.state.nj.us/rtkhsfs/chemicalsearch.aspx
Pennsylvania (P.L. 734, No. 159 and 34 Pa. Code § 323)
https://www.pacode.com/secure/data/034/chapter323/chap323toc.html
Chemicals of High Concern to
Children
Minnesota (Toxic Free Kids Act Minn. Stat. 116.9401 to 116.9407)
https://www.health.state.mn.us/communities/environment/childenvhea
lth/tfka/highconcern.html
Oregon (Toxic-Free Kids Act, Senate Bill 478, 2015)
https://www.oregon.gov/oha/PH/HEALTHYENVIRONMENTS/HEA
LTHYNEIGHBORHOODS/TOXICSUBSTANCES/Pages/childrens-
chemicals-of-concern.aspx
Vermont (18 V.S.A § 1776)
http://www.healthvermont.gov/sites/default/files/documents/2016/11/
Env_CDP_chemicals_of_high_concern_to_children.pdf
Washington State (Wash. Admin. Code 173-334-130)
https://ecology.wa.gov/Regulations-Permits/Reporting-
requirements/Reporting-for-Childrens-Safe-Products-Act/Chemicals-
of-high-concern-to-children
Other
California Candidate Chemical under Safer Consumer Products
Program (Health and Safety Code § 25252 and 25253)
https://dtsc.ca.gov/scp/candidate-chemicals-list/
California designated priority chemical for biomonitoring (California
SB 1379)
https://biomonitoring.ca.gov/sites/default/files/downloads/Designated
ChemicalsList_October2017.pdf
89
International Laws and Regulations
Table_Apx D-3 Regulatory Actions by other Governments, Tribes, and International Agreements
Country/
Organization
Requirements and Restrictions
Canada
TPP is on the Domestic Substances List (Government of Canada.
Managing substances in the environment. Substances search. Database
https://pollution-waste.canada.ca/substances-search/Substance?lang=en
European Union
TPP is registered for use in the EU. European Chemicals Agency
(ECHA) database.
TPP was evaluated under the 2017 Community Rolling Action Plan
(CoRAP) under regulation (European Commission [EC]) No1907/2006
- REACH (Registration, Evaluation, Authorisation and Restriction of
Chemicals). Additional information was requested and is due August
2020. https://echa.europa.eu/information-on-chemicals,
Australia
TPP was assessed under Human Health Tier II of the Inventory Multi-
Tiered Assessment and Prioritisation (IMAP). Uses reported include: in
plastic products, in construction materials, in cellulose acetate films, in
lubricants and transmission oils, as an industrial sealant, as a plasticizer,
as a flame retardant, in nail polishes and enamels; in manicuring
preparations, in indoor and outdoor adhesives and sealants, in coatings,
lacquers, and varnishes; in paints and inks, in roofing paper, in
polyurethane foam, in plastics and rubber, in electronic products, in
textiles, and in hydraulic fluids and lubricants. The chemical is reported
to be present in foam-based furniture and baby products (Stapleton et
al., 2011; Stapleton et al., 2009) NICNAS, 2016, Human Health Tier II
assessment for Phosphoric acid, triphenyl ester
Japan
TPP is regulated in Japan under the following legislation:
• Act on the Evaluation of Chemical Substances and Regulation of
Their Manufacture, etc. (Chemical Substances Control Law;
• CSCL) (National Institute of Technology and Evaluation
• Act of Confirmation, etc. of Release Amounts of Specific
Chemical Substances in the Environment and Promotion of
Improvements to the Management Thereof;
• Industrial Safety and Health Act (ISHA)
https://www.nite.go.jp/en/chem/chrip/chrip_search/srhInput
National Institute of Technology and Evaluation [NITE] Chemical Risk
Information Platform [CRIP]
90
Country/
Organization
Requirements and Restrictions
Basel Convention
Organic phosphorus compounds are listed as a category of waste under
the Basel Convention. Although the United States is not currently a
party to the Basel Convention, this treaty still affects U.S. importers and
exporters.
https://www.unece.org/fileadmin/DAM/stats/documents/ece/ces/ge.33/2
012/mtg1/Basel_convention_Article_1_and_Annexes.pdf
OECD Control of
Transboundary
Movements of
Wastes Destined for
Recovery Operations
Organic phosphorus compounds are listed as a category of constituents
of waste subject to The Amber Control Procedure under Council
Decision C (2001) 107/Final.
https://legalinstruments.oecd.org/en/instruments/OECD-LEGAL-0266
Australia, Austria,
Belgium, Canada,
Denmark, France,
Finland, Ireland,
New Zealand,
Romania, Singapore,
South Korea, Spain,
Switzerland, United
Kingdom
Occupational exposure limits for TPP ((GESTIS International limit
values for chemical agents (Occupational exposure limits, OELs)
database. http://limitvalue.ifa.dguv.de/WebForm_gw2.aspx

91
PROCESS, RELEASE AND OCCUPATIONAL
EXPOSURE INFORMATION
This appendix provides information and data found in preliminary data gathering for TPP.
Process Information
Process-related information potentially relevant to the risk evaluation may include process diagrams,
descriptions and equipment. Such information may inform potential release sources and worker
exposure activities.
E.1.1 Manufacturing (Including Import)
E.1.1.1 Domestic Manufacture
TPP is prepared by reacting phosphorus pentoxide and phenol and by reaction of triethyl phosphate and
chloramine-T. On a larger scale phosphorus oxychloride and phenol are reacted in an esterification tank
with heating. The hydrogen chloride formed is trapped and condensed, while the crude triphenyl
phosphate runs into a large tank where it is purified (Snyder, 1990).
E.1.1.2 Import
EPA expects that imported chemicals are often stored in warehouses prior to distribution for further
processing and use. In some cases, the chemicals may be repackaged into differently sized containers,
depending on customer demand, and QC samples may be taken for analyses (U.S. EPA, 2018b).
E.1.2 Processing and Distribution
E.1.2.1 Incorporation into a Formulation, Mixture or Reaction Product
Incorporation into a formulation, mixture, or reaction product refers to the process of mixing or blending
of several raw materials to obtain a single product or preparation. TPP may undergo several processing
steps and the processing is dependent on its downstream incorporation into articles, which is discussed
in the next subsection (U.S. EPA, 2018c).
E.1.2.2 Incorporation into an Article
Incorporation into an article typically refers to a process in which a chemical becomes an integral
component of an article (as defined at 40 CFR 704.3) for distribution in commerce. Exact process
operations involved in the incorporation of TPP-containing formulations or reaction products are
dependent on the article (U.S. EPA, 2018c). For example, TPP may be incorporated into plastics
products as a plasticizer (U.S. EPA, 2019a). EPA plans to further investigate the use of TPP being
incorporated into articles during risk evaluation.
E.1.2.3 Recycling
EPA did not identify TPP-specific information for recycling at this time; however, this chemical has
been identified in articles that are commonly recycled such as insulation, plastics and electronic
materials. The processes for recycling these materials may include grinding, washing, and rinsing the
recycled material and incorporating it into new formulations. Electronics waste recycling may involve
recovery of plastics through similar recycling processes, which are described more generally in Weil
(2001). EPA has not identified specific worker activities related to the recycling TPP-containing
products. Based on EPA’s knowledge, worker activities are anticipated to be exposed to TPP from
reclamation activities such as sorting, materials grinding steps and loading recovered materials into
transport containers.
92
E.1.3 Uses
E.1.3.1 Paints and Coatings
Based on 2019 CDR data, TPP may be used in various paints and coatings for industrial, commercial
and consumer applications. Typical process descriptions and worker activities for industrial and
commercial uses in coating applications include manual application with roller or brush, air spray
systems, airless and air-assisted airless spray systems, electrostatic spray systems,
electrodeposition/electrocoating and auto deposition, dip coating, curtain coating systems, roll coating
systems and supercritical carbon dioxide systems (U.S. EPA, 2018d; OECD, 2009).
E.1.3.2 Plastic and Rubber Products
The plastics manufacturing industry can be divided into three distinct phases: manufacturing of
polymers and chemical additives, compounding of polymer resins and chemical additives, and
converting of the compounded plastic into finished products. Compounders receive the polymer resins
from these manufacturers and produce master batches of plastics with specific properties by blending the
polymer with plastics additives (e.g., fillers, reinforcements). Converters receive the master batch of
plastics from compounders and convert it into the finished plastic product. Compounding and converting
can take place at the same facility (i.e., “in-house” manufacturing) or at separate facilities (U.S. EPA,
2014a).
E.1.3.3 Laboratory Chemicals
TPP is used as a laboratory chemical, such as in a chemical standard mixture. A commenter (EPA-HQ-
OPPT-2018-0458-0034) provided descriptions of their use of TPP in analytical standard, research,
equipment calibration and sample preparation applications, including reference sample for analysis of
terrestrial and extraterrestrial material samples, which the commenter also indicated was a critical use,
further informing EPA’s understanding of this condition of use.
E.1.3.4 Operational Fluids, Maintenance Fluids and Semisolids, Reactive Fluids,
and Solids Used in Aerospace Industry
Based on a comment received from the Aerospace Industries Association (AIA), TPP is used in
operational fluids, maintenance fluids and semisolids, reactive fluids, and solids used in the aerospace
industry. Specific uses of TPP include, but are not limited to: penetrants used for non-destructive
inspection, hydraulic fluids, engine and transmission oils, edge-filling and potting compounds, epoxy
adhesives for bonding inserts in honeycomb sandwich panels, ducts and construction of structural
composite parts, leveling compounds to assist in drainage, lubricants for bending and swaging
aluminum, titanium and corrosion resistant steel (CRES) tubes and ducts, flexible wing coatings, heat
resistant secondary fuel barriers, specialty foams for insulation and microwave absorption, landing gear
greases, and oils and lubricants (EPA-HQ-OPPT-2018-0458-0004). Based on a comment received from
National Aeronautics and Space Administration (NASA), uses of TPP also include component of
hydraulic fluid for aircraft (military specification), penetrant for non-destructive evaluation of
equipment, human-rated space flight hardware, and other high-performance components, and
component of epoxy potting material used in honeycomb panels to mount inserts for satellite structural
connections, scientific instruments, and electrical/thermal components, which the commenter also
indicated were critical uses (EPA-HQ-OPPT-2018-0458-0034).

93
E.1.3.5 Turbine Engine Oils Used in Aviation
Based on a comment received from NYCO America, LLC, TPP is used in turbine engine oils in the
aviation industry. Specifically, TPP is incorporated as an anti-wear additive in aviation turbine oils for
commercial and defense aviation jet turbines (EPA-HQ-OPPT-2018-0458-0004).
E.1.3.6 Turbine Engine Oils Used in Non-Aviation Industries
Based on a comment received from NYCO America, LLC, TPP is used in turbine engine oils in non-
aviation industries. Specifically, TPP is incorporated into aviation turbine oils used in aeroderivative gas
turbine engines (AGTs) which have applications including certain electric power generation operations
(onshore peaking and intermittent purposes, and offshore on ships and oil drilling and production
platforms) and motive power for military ships and tanks (EPA-HQ-OPPT-2018-0458-0004).
E.1.3.7 Foam Seating and Bedding Products
CDR Data indicate that TPP is used in foam seating and bedding products (U.S. EPA, 2019a). However,
specific TPP-containing foam seating and bedding products are unknown. EPA plans further investigate
the specific foam seating and bedding product use activities of TPP during the risk evaluation.
E.1.3.8 Furniture and Furnishings
CDR Data indicate that TPP is used in furniture and furnishings (U.S. EPA, 2019a). However, specific
uses of TPP in furniture and furnishings are unknown. EPA plans further investigate the use of TPP in
furniture and furnishings during this risk evaluation.
E.1.3.9 Lubricants and Greases
CDR Data indicate that TPP is used in lubricants and greases (U.S. EPA, 2019a). Based on a comment
received from NASA, TPP is a component of common off the shelf lubricants for maintenance of
overhead cranes and other equipment (EPA-HQ-OPPT-2018-0458-0034). EPA plans further investigate
the use of TPP in lubricants and greases during this risk evaluation and develop appropriate models and
approaches to estimate the exposure and releases.
E.1.3.10 Electrical and Electronic Products
CDR Data indicate that TPP is used in electrical and electronic products (U.S. EPA, 2019a). EPA plans
further investigate the use of TPP in electrical and electronic products during this risk evaluation.
E.1.4 Disposal
Disposal of a chemical should take into consideration the chemical’s potential impact on air quality,
migration to groundwater, effect on biological species, and disposal regulations (if any) (ATSDR, 2017).
Currently, TPP is not regulated as a hazardous waste. However, TPP may be disposed of as a hazardous
waste if it is present in or co-mingled with solvent mixtures that are Resource Conservation and
Recovery Act (RCRA) regulated substances.
Demolished building materials are classified as Construction and Demolition (C&D) waste, which may
be disposed in municipal solid waste landfills (MSWLFs) or C&D landfills (U.S. EPA, 2014b).
Preliminary Occupational Exposure Data
EPA presents below examples of occupational exposure-related information from the preliminary data
gathering. EPA plans consider this information and data in combination of other data and methods for
94
use in the risk evaluation. Note there are no OSHA Chemical Exposure and Health Data (CEHD) or
NIOSH Health Hazard Evaluations for TPP within the last ten years.
Table_Apx E-1. Potentially Relevant Data Sources for Exposure Monitoring and Area Monitoring
Data from NIOSH Health Hazard Evaluations for TPP
a
Year of Publication
Report Number
Facility Description
1985
HETA-83-156-1622
Plastics manufacturing facility
a
Table includes HHEs identified to date
HHEs can be found at https://www.cdc.gov/niosh/hhe/.
95
SUPPORTING INFORMATION – CONCEPTUAL MODEL FOR INDUSTRIAL
AND COMMERCIAL ACTIVITIES AND USES
Table_Apx F-1. Worker and Occupational Non-User Exposure Conceptual Model Supporting Table
Life Cycle
Stage
Category
Subcategory
Release /
Exposure
Scenario
Exposure
Pathway
Exposure
Route
Receptor /
Population
Plans to
Evaluate
Rationale
Manufacture
Manufacturing
Manufacturing
Manufacture
via reaction of
phosphorus
pentoxide/phos
phorus
oxychloride
and phenol; via
reaction of
triethyl
phosphate and
chloramine-T
Liquid
Contact
Dermal
Workers
Yes
According to CDR, all domestically
manufactured TPP is in liquid form
(suspended in solution, 30-60%
concentration), so dermal exposure to TPP
suspended in liquid will occur.
Solid
Contact
Dermal
Workers
No
According to CDR, all domestically
manufactured TPP is in liquid form
(suspended in solution, 30-60%
concentration). In addition, EPA has
identified that the processes for
manufacturing TPP involve the presence of
solution throughout the operation; thus,
dermal exposure to solid phase TPP is not
expected to be a significant exposure
pathway for TPP manufacturing.
Vapor, Mist,
Dust
Inhalation
Workers,
ONU
No
Due to the volatility of TPP (VP =2.00*10^ -
6 Torr) at room temperature, inhalation
exposure to TPP in the vapor phase is not
expected. Mist generation is not expected
during the manufacturing process. Because
the manufacturing operation for TPP
typically involves TPP suspended in
solution, dust generation is not expected
during the manufacturing process.
Liquid,
Solid
Contact
Dermal
ONU
No
Dermal exposure by ONU is not expected for
this condition of use as they are not expected
to directly handle the chemical.
96
Life Cycle
Stage
Category
Subcategory
Release /
Exposure
Scenario
Exposure
Pathway
Exposure
Route
Receptor /
Population
Plans to
Evaluate
Rationale
Import
Import
Repackaging
Liquid
Contact
Dermal
Workers
Yes
According to CDR, multiple submitters
indicated that they import TPP in liquid
form. EPA interprets this as solid TPP
suspended in solution. Exposure will occur if
the imported material is repackaged
Solid
Contact
Dermal
Workers
Yes
According to CDR, multiple submitters
indicated that they imported TPP in solid
form. Exposure will occur if the imported
material is repackaged
Vapor, Mist
Inhalation
Workers,
ONU
No
Due to the volatility of TPP (VP =2.00*10^ -
6 Torr) at room temperature, inhalation
exposure to TPP in the vapor phase is not
expected. Mist generation is not expected
during the import (i.e. repackaging) process.
Dust
Inhalation
Workers,
ONU
Yes
According to CDR, multiple submitters
indicated that they imported TPP in the form
of large crystal pellets or other solid forms.
Exposure will occur if the imported material
is repackaged.
Liquid,
Solid
Contact
Dermal
ONU
No
Dermal exposure by ONU is not expected for
this condition of use as they are not expected
to directly handle the chemical.
97
Life Cycle
Stage
Category
Subcategory
Release /
Exposure
Scenario
Exposure
Pathway
Exposure
Route
Receptor /
Population
Plans to
Evaluate
Rationale
Processing
Incorporated
into
Formulation,
Mixture, or
Reaction
Product
Flame retardant
in: All other
chemical
product and
preparation
manufacturing;
Computer and
electronic
product
manufacturing;
Plastic product
manufacturing;
Rubber product
manufacturing;
Textiles,
apparel, and
leather
manufacturing;
Utilities;
Furniture and
related product
manufacturing;
Operational
fluids,
maintenance
fluids and
semisolids,
reactive fluids,
and solids used
in aerospace
industry;
Turbine engine
oils in aviation;
Turbine engine
oils in non-
aviation
industries; and
Lubricants and
greases
Unloading/tran
sfer to mix
tanks/product
packaging
Liquid
Contact
Dermal
Workers
Yes
The potential for exposures to workers exists
during unloading and packaging operations
as TPP can be used/transported in liquid
form (suspended in solution, 30-60%)
(according to CDR data).
Solid
Contact
Dermal
Workers
Yes
The potential for exposures to workers exists
during as TPP can be used/transported in
various solid forms (according to CDR data)
Vapor, Mist
Inhalation
Workers,
ONU
No
Due to the volatility of TPP (VP =2.00*10^ -
6 Torr) at room temperature, inhalation
exposure to TPP in the vapor phase is not
expected. Mist generation is not expected
during unloading and transfer operations.
Dust
Inhalation
Workers,
ONU
Yes
Dust generation is expected during unloading
and transfer operations as TPP can be
used/transported in various solid forms
(according to CDR data)
Liquid,
Solid
Contact
Dermal
ONU
No
Dermal exposure by ONU is not expected for
this condition of use as they are not expected
to directly handle the chemical.
98
Life Cycle
Stage
Category
Subcategory
Release /
Exposure
Scenario
Exposure
Pathway
Exposure
Route
Receptor /
Population
Plans to
Evaluate
Rationale
Processing
Incorporated
into
Formulation,
Mixture, or
Reaction
Product
Paint and
coating
manufacturing
Unloading/tran
sfer to mix
tanks/product
packaging
Liquid
Contact
Dermal
Workers
Yes
The potential for exposures to workers exists
during unloading and transfer operations as
TPP can be used/transported in liquid form
(suspended in solution, 30-60%) (according
to CDR data).
Solid
Contact
Dermal
Workers
Yes
The potential for exposures to workers exists
during unloading and transfer operations as
TPP can be used/transported in various solid
forms (according to CDR data)
Vapor, Mist
Inhalation
Workers,
ONU
No
Due to the volatility of TPP (VP =2.00*10^ -
6 Torr) at room temperature, inhalation
exposure to TPP in the vapor phase is not
expected. Mist generation is not expected
during unloading or paint and coating
manufacturing processes
Dust
Inhalation
Workers,
ONU
Yes
Dust generation is expected during unloading
operations as TPP can be used/transported in
various solid forms (according to CDR data)
Liquid,
Solid
Contact
Dermal
ONU
No
Dermal exposure by ONU is not expected for
this condition of use as they are not expected
to directly handle the chemical.
Processing
Incorporated
into
Formulation,
Mixture, or
Reaction
Product
Plasticizer,
additive and
impurity in
adhesives,
sealants and
lubricants
Unloading/tran
sfer to process
equipment/
product
manufacturing
Liquid
Contact
Dermal
Workers
Yes
The potential for exposures to workers exists
during unloading and transfer operations as
TPP can be used/transported in liquid form
(suspended in solution, 30-60%) (according
to CDR data).
Solid
Contact
Dermal
Workers
Yes
The potential for exposures to workers exists
during unloading and transfer operations as
TPP can be used/transported in various solid
forms (according to CDR data)
Vapor, Mist
Inhalation
Workers,
ONU
No
Due to the volatility of TPP (VP =2.00*10^ -
6 Torr) at room temperature, inhalation
exposure to TPP in the vapor phase is not
expected. Mist generation is not expected
during unloading operations.
99
Life Cycle
Stage
Category
Subcategory
Release /
Exposure
Scenario
Exposure
Pathway
Exposure
Route
Receptor /
Population
Plans to
Evaluate
Rationale
Dust
Inhalation
Workers,
ONU
Yes
Dust generation is expected during unloading
and transfer operations as TPP can be
used/transported in various solid forms
(according to CDR data)
Liquid,
Solid
Contact
Dermal
ONU
No
Dermal exposure by ONU is not expected for
this condition of use as they are not expected
to directly handle the chemical.

100
Life Cycle
Stage
Category
Subcategory
Release /
Exposure
Scenario
Exposure
Pathway
Exposure
Route
Receptor /
Population
Plans to
Evaluate
Rationale
Processing
Incorporated
into article
Plasticizer used
in plastics
product
manufacturing
Unloading and
plastics
converting
Liquid
Contact
Dermal
Workers
Yes
The potential for exposures to workers exists
during unloading operations, as TPP can be
used/transported in liquid form (suspended in
solution, 30-60%) (according to CDR data).
Solid
Contact
Dermal
Workers
Yes
The potential for exposures to workers exists
during unloading operations as TPP can be
used/transported in various solid forms
(according to CDR data), and product
handling
Vapor, Mist
Inhalation
Workers,
ONU
No
Due to the volatility of TPP (VP =2.00*10^ -
6 Torr) at room temperature, inhalation
exposure to TPP in the vapor phase is not
expected. Mist generation is not expected
during unloading operations
Dust
Inhalation
Workers,
ONU
Yes
Dust generation is expected during unloading
operations, as TPP can be used/transported in
various solid forms (according to CDR data),
and in product finishing operations
Liquid,
Solid
Contact
Dermal
ONU
No
Dermal exposure by ONU is not expected for
this condition of use as they are not expected
to directly handle the chemical.
Processing
Incorporated
into article
Flame retardant
in: plastic
material and
resin
manufacturing;
and furniture
and related
product
manufacturing
Unloading and
plastics
converting
Liquid
Contact
Dermal
Workers
Yes
The potential for exposures to workers exists
during unloading operations, as TPP can be
used/transported in liquid form (suspended in
solution, 30-60%) (according to CDR data).
Solid
Contact
Dermal
Workers
Yes
The potential for exposures to workers exists
during unloading operations as TPP can be
used/transported in various solid forms
(according to CDR data), and product
handling
Vapor, Mist
Inhalation
Workers,
ONU
No
Due to the volatility of TPP (VP =2.00*10^ -
6 Torr) at room temperature, inhalation
exposure to TPP in the vapor phase is not
expected. Mist generation is not expected
during unloading operations
101
Life Cycle
Stage
Category
Subcategory
Release /
Exposure
Scenario
Exposure
Pathway
Exposure
Route
Receptor /
Population
Plans to
Evaluate
Rationale
Dust
Inhalation
Workers,
ONU
Yes
Dust generation is expected during unloading
operations, as TPP can be used/transported in
various solid forms (according to CDR data),
and in product finishing operations
Liquid,
Solid
Contact
Dermal
ONU
No
Dermal exposure by ONU is not expected for
this condition of use as they are not expected
to directly handle the chemical.
Processing
Recycling
Recycling
Recycling
Liquid
Contact
Dermal
Workers
Yes
The potential for exposures to workers exists
during recycling, as TPP can be incorporated
in different liquid products
Solid
Contact
Dermal
Workers
Yes
The potential for exposures to workers exists
during recycling, as TPP can be incorporated
in different solid products
Vapor, Mist
Inhalation
Workers,
ONU
No
Due to the volatility of TPP (VP =2.00*10^ -
6 Torr) at room temperature, inhalation
exposure to TPP in the vapor phase is not
expected. Mist generation is not expected
during recycling processes.
Dust
Inhalation/De
rmal
Workers
Yes
Dust exposure is expected during recycling,
as particulates from solid products
containing TPP can be generated
Liquid/
Solid
Contact
Dermal
ONU
No
Dermal exposure by ONU is not expected for
this condition of use as they are not expected
to directly handle the chemical.
Industrial,
Commercial,
Use
Foam Seating
and Bedding
Products
e.g. foam and
upholstery,
plasticizer in
automobile
upholstery
Foam handling
and product
assembly
Liquid
Contact
Dermal
Workers
No
TPP and TPP-containing article components
are not expected to be handled or used in the
liquid form.
Solid
Contact
Dermal
Workers
Yes
The potential for exposures to workers exists
during this use (Foam Seating and Bedding
Products), during the handling of foam and
manufacture of products
Vapor, Mist
Inhalation
Workers,
ONU
No
Due to the volatility of TPP (VP =2.00*10^ -
6 Torr) at room temperature, inhalation
exposure to TPP in the vapor phase is not
expected.
102
Life Cycle
Stage
Category
Subcategory
Release /
Exposure
Scenario
Exposure
Pathway
Exposure
Route
Receptor /
Population
Plans to
Evaluate
Rationale
Dust
Inhalation
Workers,
ONU
Yes
Dust generation is expected during this use
(Foam Seating and Bedding Products), as
TPP-containing articles may need to be cut
during finishing operations.
Liquid/
Solid
Contact
Dermal
ONU
No
Dermal exposure by ONU is not expected for
this condition of use as they are not expected
to directly handle the chemical.
Industrial,
Commercial,
Use
Plastic and
Rubber
Products, Not
Covered
Elsewhere
Plastic and
Rubber Products
Use of Plastic
and Rubber
products
Liquid
Contact
Dermal
Workers
No.
TPP and TPP-containing article components
are not expected to be handled or used in the
liquid form.
Solid
Contact
Dermal
Workers
Yes
The potential for exposures to workers exists
during this use (Plastic and Rubber Products,
Not Covered Elsewhere).
Vapor, Mist
Inhalation
Workers,
ONU
No
Due to the volatility of TPP (VP =2.00*10^ -
6 Torr) at room temperature, inhalation
exposure to TPP in the vapor phase is not
expected. Mist generation is not expected
during this use (Plastic and Rubber Products,
Not Covered Elsewhere).
Dust
Inhalation
Workers,
ONU
Yes
Dust generation is expected during this use
(Plastic and Rubber Products, Not Covered
Elsewhere).
Liquid/
Solid
Contact
Dermal
ONU
No
Dermal exposure by ONU is not expected for
this condition of use as they are not expected
to directly handle the chemical.
103
Life Cycle
Stage
Category
Subcategory
Release /
Exposure
Scenario
Exposure
Pathway
Exposure
Route
Receptor /
Population
Plans to
Evaluate
Rationale
Industrial,
Commercial,
Use
Paints and
Coatings
Paints and
Coatings
Unloading/
Spray Coating
Applications
Liquid
Contact
Dermal
Workers
Yes
The potential for exposures to workers exists
during unloading and application of paints
and coatings containing TPP.
Solid
Contact
Dermal
Workers
No
Paints and coatings containing TPP are not
expected to be handled or used as solids.
Vapor
Inhalation
Workers,
ONU
No
Due to the volatility of TPP (VP =2.00*10^ -
6 Torr) at room temperature, inhalation
exposure to TPP in the vapor phase is not
expected.
Mist
Inhalation
Workers,
ONU
Yes
The potential for exposure to TPP suspended
in mist exists during spray coating
applications (Paints and Coatings)
Dust
Inhalation
Workers,
ONU
No
Handling and use of paints and coatings is
not expected to generate dust.
Liquid/Solid
Contact
Dermal
ONU
No
Dermal exposure by ONU is not expected for
this condition of use as they are not expected
to directly handle the chemical.
Industrial,
Commercial,
Use
Lubricants and
Greases
Lubricants and
Greases
Use of
lubricants and
greases
Liquid
Contact
Dermal
Workers
Yes
The potential for exposures to workers exists
during the use of Lubricants and Greases
Solid
Contact
Dermal
Workers
No
Lubricants and greases containing TPP are
not expected to be handled or used as solids.
Vapor, Mist
Inhalation
Workers,
ONU
Yes
Due to the volatility of TPP (VP =2.00*10^ -
6 Torr) at room temperature, inhalation
exposure to TPP in the vapor phase is not
expected. Mist generation is possible during
the use of some Lubricants and Greases.
Dust
Inhalation
Workers,
ONU
No
Handling and use of lubricants and greases is
not expected to generate dust.
Liquid/Solid
Contact
Dermal
ONU
No
Dermal exposure by ONU is not expected for
this condition of use as they are not expected
to directly handle the chemical.
104
Life Cycle
Stage
Category
Subcategory
Release /
Exposure
Scenario
Exposure
Pathway
Exposure
Route
Receptor /
Population
Plans to
Evaluate
Rationale
Industrial,
Commercial,
Use
Electrical and
Electronic
Products
Electrical and
Electronic
Products
Use of
Electrical and
electronic
products
Liquid
Contact
Dermal
Workers
No
TPP and TPP-containing article components
are not expected to be handled or used in the
liquid form.
Solid
Contact
Dermal
Workers
Yes
The potential for exposures to workers exists
during the use and handling of Electrical and
Electronic Products
Vapor, Mist
Inhalation
Workers,
ONU
No
Due to the volatility of TPP (VP =2.00*10^ -
6 Torr) at room temperature, inhalation
exposure to TPP in the vapor phase is not
expected. Mist generation is not expected
during this use (Electrical and Electronic
Products).
Dust
Inhalation
Workers,
ONU
No
Dust generation is not expected during the
manufacture or use of Electrical and
Electronic Products.
Liquid/Solid
Contact
Dermal
ONU
No
Dermal exposure by ONU is not expected for
this condition of use as they are not expected
to directly handle the chemical.
Industrial,
Commercial,
Use
Furniture and
Furnishings,
Not Covered
Elsewhere
Furniture and
Furnishings,
Not Covered
Elsewhere
Use of
furniture and
furnishings
Liquid
Contact
Dermal
Workers
No
TPP and TPP-containing article components
are not expected to be handled or used in the
liquid form.
Solid
Contact
Dermal
Workers
Yes
The potential for exposures to workers exists
during the manufacture of Furniture and
Furnishings, Not Covered Elsewhere
Vapor, Mist
Inhalation
Workers,
ONU
No
Due to the volatility of TPP (VP =2.00*10^ -
6 Torr) at room temperature, inhalation
exposure to TPP in the vapor phase is not
expected. Mist generation is not expected
during this use (Furniture and Furnishings,
Not Covered Elsewhere).
Dust
Inhalation
Workers,
ONU
Yes
Dust generation is expected during the
manufacture of Furniture and Furnishings,
Not Covered Elsewhere
Liquid/Solid
Contact
Dermal
ONU
No
Dermal exposure by ONU is not expected for
this condition of use as they are not expected
to directly handle the chemical.
Liquid
Contact
Dermal
Workers
Yes
TPP is expected to be in liquid form in
operational fluids and reactive fluids
105
Life Cycle
Stage
Category
Subcategory
Release /
Exposure
Scenario
Exposure
Pathway
Exposure
Route
Receptor /
Population
Plans to
Evaluate
Rationale
Industrial,
Commercial,
Use
Operational
fluids,
maintenance
fluids and
semisolids,
reactive fluids,
and solids used
in aerospace
industry
Operational
fluids,
maintenance
fluids and
semisolids,
reactive fluids,
and solids used
in aerospace
industry
Use of
operational
fluids,
maintenance
fluids and
semisolids,
reactive fluids,
and solids used
in aerospace
industry
Solid
Contact
Dermal
Workers
Yes
The potential for exposures to workers exists
during the use of semisolids and solids in the
aerospace industry.
Vapor, Mist
Inhalation
Workers,
ONU
No
Due to the volatility of TPP (VP =2.00*10^ -
6 Torr) at room temperature, inhalation
exposure to TPP in the vapor phase is not
expected. Mist generation is not expected
during this use (Operational fluids,
maintenance fluids and semisolids, reactive
fluids, and solids used in aerospace industry).
Dust
Inhalation
Workers,
ONU
Yes
Dust generation is expected during the use of
solids and semisolids in the aerospace
industry.
Liquid/Solid
Contact
Dermal
ONU
No
Dermal exposure by ONU is not expected for
this condition of use as they are not expected
to directly handle the chemical.
Industrial,
Commercial,
Use
Turbine engine
oils in aviation
Turbine engine
oils in aviation
Use of turbine
engine oils
Liquid
Contact
Dermal
Workers
Yes
TPP in turbine engine oils is expected to be
used in liquid form
Solid
Contact
Dermal
Workers
No
TPP is not expected to be in solid form when
used in turbine engine oils
Vapor, Mist
Inhalation
Workers,
ONU
Yes
Due to the volatility of TPP (VP =2.00*10^ -
6 Torr) at room temperature, inhalation
exposure to TPP in the vapor phase is not
expected. Mist generation is possible during
this use (Turbine engine oils in aviation).
Dust
Inhalation
Workers,
ONU
No
Dust generation is not expected during the
use of turbine engine oils
Liquid/Solid
Contact
Dermal
ONU
No
Dermal exposure by ONU is not expected for
this condition of use as they are not expected
to directly handle the chemical.
Liquid
Contact
Dermal
Workers
Yes
TPP in turbine engine oils is expected to be
used in liquid form
106
Life Cycle
Stage
Category
Subcategory
Release /
Exposure
Scenario
Exposure
Pathway
Exposure
Route
Receptor /
Population
Plans to
Evaluate
Rationale
Industrial,
Commercial,
Use
Turbine engine
oils in non-
aviation
industries
Turbine engine
oils in non-
aviation
industries
Use of turbine
engine oils
Solid
Contact
Dermal
Workers
No
TPP is not expected to be in solid form when
used in turbine engine oils
Vapor, Mist
Inhalation
Workers,
ONU
Yes
Due to the volatility of TPP (VP =2.00*10^ -
6 Torr) at room temperature, inhalation
exposure to TPP in the vapor phase is not
expected. Mist generation is possible during
this use (Turbine engine oils in non-aviation
industries).
Dust
Inhalation
Workers,
ONU
No
Dust generation is not expected during the
use of turbine engine oils
Liquid/Solid
Contact
Dermal
ONU
No
Dermal exposure by ONU is not expected for
this condition of use as they are not expected
to directly handle the chemical.
Industrial,
Commercial,
Use
Laboratory
Chemical
Laboratory
Chemical
Use as a
laboratory
chemical
Liquid
Contact
Dermal
Workers
Yes
The potential for exposures to workers exists
during this use (Laboratory chemical), as
TPP is in liquid form.
Solid
Contact
Dermal
Workers
Yes
The potential for exposures to workers exists
during this use (laboratory chemicals), as
TPP can be used/transported in pellet or
crystal form (according to CDR)
Vapor, Mist
Inhalation
Workers,
ONU
No
Due to the volatility of TPP (VP =2.00*10^ -
6 Torr) at room temperature, inhalation
exposure to TPP in the vapor phase is not
expected. Mist generation is not expected
during this use (Laboratory chemical).
Dust
Inhalation
Workers,
ONU
Yes
Dust generation is expected during the use of
TPP as a laboratory chemical
Liquid/Solid
Contact
Dermal
ONU
No
Dermal exposure by ONU is not expected for
this condition of use as they are not expected
to directly handle the chemical.
107
Life Cycle
Stage
Category
Subcategory
Release /
Exposure
Scenario
Exposure
Pathway
Exposure
Route
Receptor /
Population
Plans to
Evaluate
Rationale
Disposal
Waste Handling,
Treatment and
Disposal
Disposal of TPP
containing
wastes
Worker
handling of
wastes
Solid
Contact
Dermal
Workers
Yes
Dermal exposure is expected for this
condition of use.
Dust
Inhalation
Workers
Yes
TPP is solid at room temperature, EPA plans
to evaluate the inhalation pathway.
Liquid
Contact
Dermal
ONU
No
Dermal exposure by ONU is not expected for
this condition of use as they are not expected
to directly handle the chemical.
Dust
Inhalation
ONU
Yes
TPP is solid at room temperature, EPA plans
to evaluate the inhalation pathway.
108
SUPPORTING INFORMATION- CONCEPTUAL MODEL FOR CONSUMER
ACTIVITIES AND USES
Table_Apx G-1. Consumer Exposure Conceptual Model Supporting Table
Life Cycle
Stage
Category
Subcategory
Release from
source
Exposure
Pathway
Exposure
Route
Receptor
Plans to
Evaluate
Rationale
Consumer Use
Lubricants and
greases
Hydraulic fluids
Direct contact
through use of
products/articles
containing TPP
Air/Particulate
Inhalation
Consumers/
Bystanders
Yes
Inhalation via air and/or particulate
exposure may occur during
product/article use. EPA plans to
analyze inhalation exposure.
Article/Product
Contact
Dermal
Consumers
Yes
Dermal exposure may occur via use
of articles containing TPP. EPA
plans to analyze dermal exposure.
Air/Particulate
Inhalation
Consumers
and
Bystanders
Yes
Inhalation of air and/or particles
from articles/products containing
TPP may occur for this condition of
use. EPA plans to analyze inhalation
exposure.
Consumer Use
Electrical and
electronic
records
Electrical and
electronic
records
Direct contact
through use of
products/articles
containing TPP
Air/Particulate
Inhalation
Consumers/
Bystanders
Yes
Inhalation via air and/or particulate
exposure may occur during
product/article use. EPA plans to
analyze inhalation exposure.
Article/Product
Contact
Dermal
Consumers
Yes
Dermal exposure may occur via use
of articles containing TPP. EPA
plans to analyze dermal exposure.
Air/Particulate
Inhalation
Consumers
and
Bystanders
Yes
Inhalation of air and/or particles
from articles/products containing
TPP may occur for this condition of
use. EPA plans to analyze inhalation
exposure.
Dust
Ingestion
Consumers
Yes
Ingestion of TPP sorbed onto dust
may occur for this condition of use.
EPA plans to analyze dust exposure
via ingestion.
109
Life Cycle
Stage
Category
Subcategory
Release from
source
Exposure
Pathway
Exposure
Route
Receptor
Plans to
Evaluate
Rationale
Consumer Use
Plastics and
rubber
products, not
covered
elsewhere
Thermoplastics
Direct contact
through use of
products/articles
containing TPP
Air/Particulate
Inhalation
Consumers/
Bystanders
Yes
Inhalation via air and/or particulate
exposure may occur during
product/article use. EPA plans to
analyze inhalation exposure.
Dust
Ingestion
Consumers
Yes
Ingestion of TPP sorbed onto dust
may occur for this condition of use.
EPA plans to analyze dust exposure
via ingestion.
Article/Product
Contact
Dermal
Consumers
Yes
Dermal exposure may occur via use
of articles containing TPP. EPA
plans to analyze dermal exposure.
Article/Product
Mouthing
Ingestion
Consumers
Yes
Ingestion via object to mouth or
subsequent hand to mouth from
product dermal contact. EPA plans
to analyze mouthing via ingestion.
Vulcanization
accelerator
Direct contact
through use of
products/articles
containing TPP
Article/Product
Contact
Dermal
Consumers
Yes
Dermal exposure may occur for this
condition of use. EPA plans to
analyze dermal exposure.
Dust
Ingestion
Consumers
Yes
Ingestion of TPP sorbed onto dust
may occur for this condition of use.
EPA plans to analyze dust exposure
via ingestion.
Air/Particulate
Inhalation
Consumers
and
Bystanders
Yes
Inhalation of air and/or particles
from articles/products containing
TPP may occur for this condition of
use. EPA plans to analyze inhalation
exposure.
Article/Product
Contact
Dermal
Consumers
Yes
Dermal exposure may occur via use
of articles containing TPP. EPA
plans to analyze dermal exposure.
Article/Product
Mouthing
Ingestion
Consumers
Yes
Ingestion via object to mouth or
subsequent hand to mouth from
product dermal contact. EPA plans
to analyze mouthing via ingestion.
Flame retardants
in camping tents
Direct contact
through use of
Air/Particulate
Inhalation
Consumers/
Bystanders
Yes
Inhalation via air and/or particulate
exposure may occur during
110
Life Cycle
Stage
Category
Subcategory
Release from
source
Exposure
Pathway
Exposure
Route
Receptor
Plans to
Evaluate
Rationale
products/articles
containing TPP
product/article use. EPA plans to
analyze inhalation exposure.
Dust
Ingestion
Consumers
Yes
Ingestion of TPP sorbed onto dust
may occur for this condition of use.
EPA plans to analyze dust exposure
via ingestion.
Article/Product
Contact
Dermal
Consumers
Yes
Dermal exposure may occur via use
of articles containing TPP. EPA
plans to analyze dermal exposure.
Article/Product
Mouthing
Ingestion
Consumers
Yes
Ingestion via object to mouth or
subsequent hand to mouth from
product dermal contact. EPA plans
to analyze mouthing via ingestion.
Consumer Use
Foam seating
and bedding
products
Foam and
upholstery
Direct contact
through use of
products/articles
containing TPP
Air/Particulate
Inhalation
Consumers/
Bystanders
Yes
Inhalation via air and/or particulate
exposure may occur during
product/article use. EPA plans to
analyze inhalation exposure.
Dust
Ingestion
Consumers
Yes
Ingestion of TPP sorbed onto dust
may occur for this condition of use.
EPA plans to analyze dust exposure
via ingestion.
Article/Product
Contact
Dermal
Consumers
Yes
Dermal exposure may occur via use
of articles containing TPP. EPA
plans to analyze dermal exposure.
Article/Product
Mouthing
Ingestion
Consumers
Yes
Ingestion via object to mouth or
subsequent hand to mouth from
product dermal contact. EPA plans
to analyze mouthing via ingestion.
Plasticizer in
automobile
upholstery
Direct contact
through use of
products/articles
containing TPP
Air/Particulate
Inhalation
Consumers/
Bystanders
Yes
Inhalation via air and/or particulate
exposure may occur during
product/article use. EPA plans to
analyze inhalation exposure.
Dust
Ingestion
Consumers
Yes
Ingestion of TPP sorbed onto dust
may occur for this condition of use.
EPA plans to analyze dust exposure
via ingestion.
111
Life Cycle
Stage
Category
Subcategory
Release from
source
Exposure
Pathway
Exposure
Route
Receptor
Plans to
Evaluate
Rationale
Article/Product
Contact
Dermal
Consumers
Yes
Dermal exposure may occur via use
of articles containing TPP. EPA
plans to analyze dermal exposure.
Article/Product
Mouthing
Ingestion
Consumers
Yes
Ingestion via object to mouth or
subsequent hand to mouth from
product dermal contact. EPA plans
to analyze mouthing via ingestion.
Consumer
Handling of
Disposal and
Waste
Wastewater,
Liquid wastes
and solid
wastes
Wastewater,
Liquid wastes
and solid wastes
Direct contact
through use of
products/articles
containing TPP
Article/Product
Contact
Dermal
Consumers
Yes
Dermal exposure may occur for this
condition of use. EPA plans to
analyze dermal exposure.
Dust
Ingestion
Consumers
Yes
Ingestion of TPP sorbed onto dust
may occur for this condition of use.
EPA plans to analyze dust exposure
via ingestion.
Air/Particulate
Inhalation
Consumers
and
Bystanders
Yes
Inhalation of air and/or particles
from articles/products containing
TPP may occur for this condition of
use. EPA plans to analyze inhalation
exposure.
Long-term
emission/mass-
transfer through
use of products
containing TPP
Dust
Ingestion
Consumers
Yes
Ingestion of TPP sorbed onto dust
may occur for this condition of use.
EPA plans to analyze dust exposure
via ingestion.
Air/Particulate
Inhalation
Consumers
and
Bystanders
Yes
Inhalation of air and/or particles
from articles/products containing
TPP may occur for this condition of
use. EPA plans to analyze inhalation
exposure.
112
SUPPORTING INFORMATION – CONCEPTUAL MODEL FOR
ENVIRONMENTAL RELEASES AND WASTES
Table_Apx H-1. General Population and Environmental Exposure Conceptual Model Supporting Table
Life Cycle
Stage
Category
Release
Exposure Pathway /
Media
Exposure
Routes
Receptor /
Population
Plans to
Evaluate
Rationale
All
Emissions
to Air
Emissions
to Air
Near facility ambient
air concentrations
Inhalation
General
Population
Yes
TPP deposition to nearby bodies of water and
soil are expected exposure pathways, not
covered under other EPA regulations, and,
therefore in scope.
Indirect deposition to
nearby bodies of
water and soil
catchments
Oral
Dermal
General
Population
Yes
TBD
Aquatic and
Terrestrial
Receptors
Yes
Wastewater
or Liquid
Wastes
Industrial
pre-
treatment
and
wastewater
treatment,
or POTW
Direct release into
surface water and
indirect partitioning
to sediment
TBD
Aquatic and
Terrestrial
Receptors
Yes
EPA plans to analyze the release of TPP into
surface water and indirect partitioning to
sediment exposure pathways to aquatic and
terrestrial receptors.
Oral
Dermal
General
Population
Yes
EPA plans to analyze the release of TPP into
surface water and indirect partitioning to
sediment and bioaccumulation exposure
pathways to the general population.
Drinking Water via
Surface or Ground
Water
Oral
Dermal and
Inhalation
(e.g.
showering)
General
Population
Yes
EPA plans to analyze the release of TPP into
surface water and indirect partitioning to
drinking water.
Biosolids: application
to soil and/or
migration to
groundwater and/or
surface water
Oral (e.g.
ingestion of
soil)
Inhalation
General
Population
Yes
EPA plans to analyze the pathway from
biosolids to the general population, aquatic and
terrestrial species.
TBD
Aquatic and
Terrestrial
Receptors
Yes
Disposal
Solid and
Liquid
Wastes
Municipal
landfill and
Leachate to soil,
ground water and/or
Oral
Dermal
General
Population
Yes
EPA plans to analyze the pathway from
municipal landfills and other land disposal to the
113
Life Cycle
Stage
Category
Release
Exposure Pathway /
Media
Exposure
Routes
Receptor /
Population
Plans to
Evaluate
Rationale
other land
disposal
migration to surface
water
TBD
Aquatic and
Terrestrial
Receptors
general population, aquatic and terrestrial
receptors.
